
Artificial intelligence in medical image analysis and molecular diagnostics: recent advances and applications
Introduction
“All models are wrong, but some are useful”—George Box.
Since its conceptual beginnings in the 1950s, AI has been rapidly transforming the landscape of healthcare, with its impact particularly pronounced in the field of medical diagnostics (1). Early diagnostic systems, like INTERNIST-1 developed in 1971, used rule-based frameworks and medical knowledge bases to assist internal medicine physicians in diagnosis (2). By 1986, more sophisticated systems emerged, such as DXplain, a decision support system that used modified Bayesian logic to generate differential diagnoses from inputted symptoms while serving as an electronic medical textbook (3). The field has since evolved from early rule-based models to computer-aided diagnosis (CAD) systems for medical imaging interpretation emerging in the 1990s (4) to today’s deep learning (DL) architectures, enabled by increased computing power, larger datasets, and sophisticated algorithms.
State-of-the-art artificial intelligence (AI) models promise to improve accuracy, speed, and accessibility in disease detection and patient care. As we stand on the cusp of a new era in molecular medicine, AI-driven diagnostic tools are emerging as powerful allies for professionals across the healthcare delivery spectrum, potentially reshaping how diseases are identified, monitored, and treated.
The integration of AI in diagnostics spans a wide range of applications, from analysing medical imaging to detecting meaningful regularities in complex genomic data. Machine learning (ML) and DL algorithms, trained on vast datasets of patient information, are demonstrating remarkable capabilities in pattern recognition and predictive analytics. These advancements are not only augmenting the capabilities of medical professionals but also opening new avenues for personalized medicine and early intervention strategies (5).
However, as with any revolutionary technology, the advent of AI in diagnostics brings both immense potential and significant challenges. Questions of accuracy, interpretability, ethics, and integration into existing healthcare systems must be carefully addressed (6). This mini-review will discuss the fundamentals of AI, explore its applications and limitations in diagnostic medicine, and appraise whether AI is indeed the panacea it’s believed to be.
AI fundamentals: the toolbox for understanding data
AI is a technology that can problem-solve and perform tasks typically requiring human intelligence. A field of computer science introduced by Alan Turing and coined by John McCarthy in 1956, AI is predicated on traditional statistical learning (7). It includes techniques such as ML and DL.
In ML, programmers train models to learn from datasets and predict target values for new data points. Supervised ML, which uses labelled data, includes techniques like linear and logistic regression—simple models applicable to diagnostics. For instance, cancer diagnosis exemplifies logistic regression. Input features (x values) may include patient demographics and tumour characteristics, while target outputs (y values) categorize tumours as benign or malignant. Logistic classification employs the gradient descent algorithm to optimize weights and biases, modelling the relationship between inputs and outputs to predict discrete categories. Chhatwal et al. developed such a model for breast cancer diagnosis using the 36 features described in the US National Mammography Database (8).
Meanwhile, unsupervised ML techniques, such as k-means cluster analysis, can use unlabelled data for the identification of novel sub-groups of medical conditions, such as diabetes and sepsis (9,10). Such stratification can help tailor treatment regimens, direct research, and identify patients at high risk for certain complications.
DL, a subset of ML, is the current industry paradigm. It uses artificial neurons in architectures inspired by the human brain, forming a neural network or multi-layer perceptron. It is used to solve problems of greater complexity and compute requirements than classical ML (11). For example, Xue et al. developed an advanced DL classifier for differential diagnosis of dementia using complex high-dimensional multimodal data, including demographics, medical history, neuroimaging, and neuropsychological assessments. It helps address the challenges of distinguishing between various dementia aetiologies, such as Alzheimer’s, Lewy body dementia, and vascular dementia. The study drew on 51,269 patients and achieved an area under the curve (AUC) of 0.96 in the model’s ability to identify dementia aetiologies (12).
The transformative potential of AI in diagnostics
Molecular diagnostics
The synergy of AI with molecular diagnostics is accelerating the pace of discovery in precision medicine and personalized healthcare. Owing to the rise of big data, the past decade has witnessed a staggering increase in the scale and complexity of medical data collected. Therefore, the most sophisticated molecular diagnostic AI systems currently use multi-dimensional omics datasets, such as genomics, metabolomics, proteomics, and transcriptomics data (13). Figure 1 demonstrates the ML development pathway for molecular diagnostics (14).
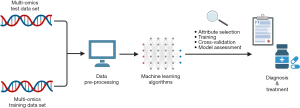
AI-driven diagnostic tools leverage the vast amounts of data generated by next-generation sequencing, enabling the analysis of complex genomic and molecular patterns for earlier and more accurate disease diagnosis (13). For example, AI is dramatically improving diagnostic capabilities in the realm of rare genetic disorders. Such novel technologies are essential given 70% of individuals seeking a diagnosis today for Mendelian disorders remain undiagnosed (15). DL models trained on large genomic databases can quickly analyse an individual’s genetic data to identify potential disease-causing variants. AI-MARRVEL and SHEPHERD represent two promising DL strategies for rare disease diagnosis and novel disease gene discovery. Both are benchmarked on real-world cohorts, with AI-MARRVEL achieving a precision rate of 98% (16,17). A 2022 multicentre study in South Korea demonstrated the clinical utility of an AI-based diagnostic program called EVIDENCE for paediatric rare genetic diseases, achieving diagnostic rates of 40–52% across different conditions while maintaining high user satisfaction among both physicians and patients. The system could analyse over 100,000 genetic variants within minutes based on patient phenotype, accelerating diagnostic workflows (18). However, these diagnostic rates highlight that AI tools must work in tandem with clinical expertise rather than as standalone solutions. While AI can dramatically speed up variant analysis and suggest potential diagnoses, physician oversight and clinical correlation remain essential for accurate diagnosis.
Another promising application is cancer diagnostics. AI tools can identify specific genetic mutations, gene expression patterns, and chromosomal abnormalities that are indicative of different cancer types and subtypes. Moreover, AI-powered liquid biopsy analysis is emerging as a non-invasive method for early cancer detection and monitoring, capable of identifying circulating tumour DNA (ctDNA) and other cancer biomarkers in blood samples. Widman et al. introduced a DL system to parse DNA sequencing data from patient blood tests and detect ctDNA with high accuracy. They used an advanced approach to differentiate patterns indicating cancer from sequencing errors and other noise. Their model can be applied for the early detection of a wide variety of cancers such as colorectal cancer, lung cancer, and melanoma (19).
Furthermore, the application of AI in microbiome analysis is opening new avenues for diagnosing various conditions, from gastrointestinal disorders to neurological diseases. By analysing metagenomic sequencing data, AI models can identify microbial signatures associated with specific diseases, offering a novel approach to diagnosis that considers the complex interactions between the human host and its microbial inhabitants. This technology is particularly promising for conditions where traditional diagnostic methods have fallen short. Park et al. are able to diagnose liver diseases using several gut microbiota-based AI models, achieving AUCs above 0.90 in external validation using diverse patient data (20).
Computer vision
Historically, the field of radiology has been the most receptive to the implementation of CAD, with CAD being approved by the US Food and Drug Administration (FDA) in 1998. CAD is routinely applied in multiple clinical settings, such as screening sites for breast cancer and hospitals for pulmonary and musculoskeletal pathologies, in countries such as the US, UK, Australia, Germany, Ireland, and the Netherlands (21-26). In radiology, AI-powered computer vision systems are transforming the interpretation of medical images such as computed tomography (CT) scans, X-rays, and magnetic resonance imaging (MRI) images. For instance, in chest radiography, AI models such as convolutional neural networks (CNNs) have shown impressive accuracy in identifying lung nodules, pneumonia, and even early signs of coronavirus disease 2019 (COVID-19) (27). Some studies have shown AI tools surpassing radiologists in diagnosing certain pathologies, particularly lung diseases. For instance, Plesner et al. demonstrated that a commercial DL model identified abnormal chest radiographs with 99.1% sensitivity, significantly outperforming human radiologists who achieved 72.3% sensitivity (28). Figure 2 compares AI-powered and traditional radiological diagnosis (29).
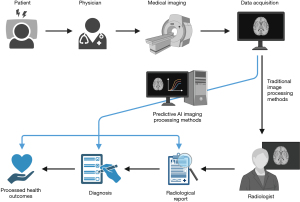
In mammography, AI assistants are helping radiologists detect breast cancer at earlier stages, potentially improving survival rates. For example, Donnelly et al. trained a DL algorithm that could predict a patient’s 1-to-5-year risk of developing breast cancer with high accuracy, by analysing the bilateral dissimilarity of breast tissue patterns in mammograms. Their model had a 1-year breast cancer risk AUC of 0.79 and a 5-year risk AUC of 0.66 (30). Such AI systems not only enhance diagnostic precision but also help prioritize urgent cases in busy clinical settings, ensuring timely interventions for critical conditions. The potential real-world impact of AI in mammography screening is now being tested in the landmark EDITH trial launched by the UK’s National Health Service (NHS) in 2025. This prospective study of 700,000 women will evaluate whether AI combined with a single radiologist can match or exceed the current standard of double reading by human specialists. The trial is particularly significant given that second readers typically identify an additional 6–10% of cancers, setting a clear benchmark for AI performance (31). While preliminary retrospective studies and smaller prospective trials have shown promise (32), EDITH will provide important evidence about AI’s effectiveness in real clinical settings, potentially addressing critical workforce shortages in radiology.
Additionally, computer vision algorithms can rapidly analyse whole-slide images (WSIs), detecting and classifying cellular abnormalities with high precision. These systems are particularly valuable in cancer diagnostics, where they can assist pathologists in grading tumours, identifying specific cancer subtypes, pre-screening high-risk patients, and even predicting treatment responses based on tissue morphology. Neto et al. describe an interpretable DL system for colorectal cancer diagnosis from WSIs, demonstrating an accuracy of 93.44% on the internal dataset and 84.91% on a challenging external dataset (33).
Beyond improving diagnostic accuracy, computer vision is addressing critical challenges in healthcare delivery. By automating routine screenings and providing rapid preliminary assessments, these systems can significantly reduce diagnostic backlogs and waiting times, especially in regions facing shortages of medical specialists. For instance, the integration of dermatological AI technologies with smartphone applications is democratizing access to skin health assessments. Leveraging widespread smartphone adoption, these tools enable preliminary skin screenings in remote or underserved areas. One such app, SkinVision, has more than 900,000 users worldwide and exhibited a sensitivity of 95.1% in detecting pre-malignant skin lesions (34).
Additionally, the consistency offered by AI-assisted diagnoses can help standardize care across different healthcare settings, potentially reducing disparities in diagnostic quality. In the field of cardiology, Mason et al. created a CNN-based model to reconstruct a 12-lead ECG using only three leads, potentially expanding access to this diagnostic tool in resource-limited environments while maintaining quality (35).
Challenges of diagnostic AI tools
Any discussion on the opportunities of AI must be tempered with consideration of its risks. The success of AI is largely predicated on the quality and representativeness of the data used to train it, as well as the algorithms used. Obtaining such data can be challenging in the medical domain due to privacy concerns and data fragmentation. Moreover, models can perpetuate biases present in the training data which can lead to certain patient populations being discriminated. A recent study by Daneshjou et al. underscores this concern in dermatology, demonstrating that state-of-the-art AI algorithms for skin lesion classification perform substantially worse on images of dark skin tones compared to light skin tones, highlighting the need for diverse datasets in medical AI development (36).
The integration of such tools into healthcare systems also raises complex legal and regulatory questions surrounding privacy, liability, and the oversight of AI-assisted diagnoses. The “black-box” nature of many DL algorithms is a particular concern as explainability is important for stakeholders to establish trust in new technologies (37). The lack of transparency of some AI systems makes it challenging to understand their diagnostic decision-making processes fully, potentially fostering doubt and unease in patient populations. The consensus, as per several patient perception surveys, is that doctors must maintain an integral oversight role and be able to override AI’s diagnoses (38).
Beyond these technical and trust challenges, healthcare systems face significant practical barriers to AI adoption, including the need for substantial infrastructure investment, workforce training, and standardized protocols for AI integration. Clinical workflows must be redesigned to effectively incorporate AI tools while maintaining human judgment as the final decision-making authority. There are also concerns about ensuring equitable access across different demographics and healthcare settings (39,40). Regulatory frameworks are still evolving to address these challenges, with agencies like the FDA developing new action plans and guidance documents for AI/ML-based medical devices (41).
Additionally, causality is rapidly gaining traction as an important property of clinical AI. Present-day ML and DL algorithms excel at making predictions and discerning patterns. However, majority of current diagnostic AI systems lack the ability to establish causal relationships with the level of certainty required for clinical applications. For instance, while molecular diagnostic AI models can reveal correlations between specific ctDNA sequences and certain types of cancer, most models cannot definitively determine the causal mechanisms by which genetic mutations in proto-oncogenes, tumour suppressor genes or mismatch repair genes lead to neoplastic changes (42,43). Clinicians consider this understanding indispensable to determining a patient’s prognosis and providing individualized treatment (44). Thus, instead of working in silos, a partnership between clinicians and AI would leverage their combined strengths to enhance healthcare systems and improve patient outcomes.
Lastly, clinical intuition, or non-analytical reasoning, is irreplaceable. One study of general practitioners noted that their “feeling ‘there is something wrong’ is the best predictor among all signs and symptoms of paediatric infections” (45). Irrespective of how many gigabytes a multimodal dataset is, there will always be crucial uncaptured informal data which healthcare professionals have access to. AI models only capture some of a patient’s story—physician oversight helps ensure its nuances aren’t lost in a blunt instrument.
Conclusions
Diagnoses, whether for flu or rare genetic disease, unequivocally have an impact on a patient’s prospect of their present and future life. AI has an expanding repertoire of applications in medical diagnostics. Aside from increased precision and diagnostic equity, such automation is advantageous as it can alleviate some of the demand on physicians’ time and mental bandwidth. This additional clinical time could allow healthcare providers to focus more on patient interaction, potentially enhancing the overall quality of care.
However, tackling challenges such as bias, data quality, transparency, and legal frameworks is essential to harnessing the power of diagnostic AI tools. As we navigate these complexities, the ultimate goal remains clear: to leverage AI’s potential in diagnostics while ensuring that it complements and enhances, rather than replaces, the irreplaceable human element in healthcare delivery.
Acknowledgments
None.
Footnote
Peer Review File: Available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-412/prf
Funding: None.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-412/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaul V, Enslin S, Gross SA. History of artificial intelligence in medicine. Gastrointest Endosc 2020;92:807-12. [Crossref] [PubMed]
- Miller RA, Pople HE Jr, Myers JD. Internist-1, an experimental computer-based diagnostic consultant for general internal medicine. N Engl J Med 1982;307:468-76. [Crossref] [PubMed]
- Barnett GO, Cimino JJ, Hupp JA, et al. DXplain. An evolving diagnostic decision-support system. JAMA 1987;258:67-74. [Crossref] [PubMed]
- Giger ML, Chan HP, Boone J. Anniversary paper: History and status of CAD and quantitative image analysis: the role of Medical Physics and AAPM. Med Phys 2008;35:5799-820. [Crossref] [PubMed]
- Kumar Y, Koul A, Singla R, et al. Artificial intelligence in disease diagnosis: a systematic literature review, synthesizing framework and future research agenda. J Ambient Intell Humaniz Comput 2023;14:8459-86. [Crossref] [PubMed]
- Kulkarni PA, Singh H. Artificial Intelligence in Clinical Diagnosis: Opportunities, Challenges, and Hype. JAMA 2023;330:317-8. [Crossref] [PubMed]
- Haenlein M, Kaplan A. A brief history of artificial intelligence: On the past, present, and future of artificial intelligence. California Management Review 2019;61:5-14. [Crossref]
- Chhatwal J, Alagoz O, Lindstrom MJ, et al. A logistic regression model based on the national mammography database format to aid breast cancer diagnosis. AJR Am J Roentgenol 2009;192:1117-27. [Crossref] [PubMed]
- Sharafoddini A, Dubin JA, Lee J. Identifying subpopulations of septic patients: A temporal data-driven approach. Comput Biol Med 2021;130:104182. [Crossref] [PubMed]
- Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018;6:361-9. [Crossref] [PubMed]
- Rahman A, Debnath T, Kundu D, et al. Machine learning and deep learning-based approach in smart healthcare: Recent advances, applications, challenges and opportunities. AIMS Public Health 2024;11:58-109. [Crossref] [PubMed]
- Xue C, Kowshik SS, Lteif D, et al. AI-based differential diagnosis of dementia etiologies on multimodal data. Nat Med 2024;30:2977-89. [Crossref] [PubMed]
- Quazi S. Artificial intelligence and machine learning in precision and genomic medicine. Med Oncol 2022;39:120. [Crossref] [PubMed]
BioRender 2024 . Available online: https://www.biorender.com/- Alsentzer E, Finlayson SG, Li MM, et al. Simulation of undiagnosed patients with novel genetic conditions. Nat Commun 2023;14:6403. [Crossref] [PubMed]
- Alsentzer E, Li MM, Kobren SN, et al. Few shot learning for phenotype-driven diagnosis of patients with rare genetic diseases. medRxiv [Preprint]. 2022 [cited 2024 Aug 16]. Available online: https://www.medrxiv.org/content/10.1101/2022.12.07.22283238v3
10.1101/2022.12.07.22283238 10.1101/2022.12.07.22283238 - Mao D, Liu C, Wang L, et al. AI-MARRVEL - A Knowledge-Driven AI System for Diagnosing Mendelian Disorders. NEJM AI 2024;1: [Crossref] [PubMed]
- Choi IH, Seo GH, Park J, et al. Evaluation of users' level of satisfaction for an artificial intelligence-based diagnostic program in pediatric rare genetic diseases. Medicine (Baltimore) 2022;101:e29424. [Crossref] [PubMed]
- Widman AJ, Shah M, Frydendahl A, et al. Ultrasensitive plasma-based monitoring of tumor burden using machine-learning-guided signal enrichment. Nat Med 2024;30:1655-66. [Crossref] [PubMed]
- Park IG, Yoon SJ, Won SM, et al. Gut microbiota-based machine-learning signature for the diagnosis of alcohol-associated and metabolic dysfunction-associated steatotic liver disease. Sci Rep 2024;14:16122. [Crossref] [PubMed]
- Doi K. Computer-aided diagnosis in medical imaging: historical review, current status and future potential. Comput Med Imaging Graph 2007;31:198-211. [Crossref] [PubMed]
- Kuo RYL, Harrison C, Curran TA, et al. Artificial Intelligence in Fracture Detection: A Systematic Review and Meta-Analysis. Radiology 2022;304:50-62. [Crossref] [PubMed]
- Pauling C, Kanber B, Arthurs OJ, et al. Commercially available artificial intelligence tools for fracture detection: the evidence. BJR Open 2023;6:tzad005. [Crossref] [PubMed]
- Eisemann N, Bunk S, Mukama T, et al. Nationwide real-world implementation of AI for cancer detection in population-based mammography screening. Nat Med 2025;31:917-24. [Crossref] [PubMed]
- Chau M. Ethical, legal, and regulatory landscape of artificial intelligence in Australian healthcare and ethical integration in radiography: A narrative review. J Med Imaging Radiat Sci 2024;55:101733. [Crossref] [PubMed]
- van Leeuwen KG, de Rooij M, Schalekamp S, et al. Clinical use of artificial intelligence products for radiology in the Netherlands between 2020 and 2022. Eur Radiol 2024;34:348-54. [Crossref] [PubMed]
- Akhter Y, Singh R, Vatsa M. AI-based radiodiagnosis using chest X-rays: A review. Front Big Data 2023;6:1120989. [Crossref] [PubMed]
- Plesner LL, Müller FC, Nybing JD, et al. Autonomous Chest Radiograph Reporting Using AI: Estimation of Clinical Impact. Radiology 2023;307:e222268. [Crossref] [PubMed]
- Ona S. Biomedical Image Analysis (AI vs Traditional Techniques). 2023. Available online: https://app.biorender.com/biorender-templates/figures/all/t-6527132578c5094993f1408a-biomedical-image-analysis-ai-vs-traditional-techniques
- Donnelly J, Moffett L, Barnett AJ, et al. AsymMirai: Interpretable Mammography-based Deep Learning Model for 1-5-year Breast Cancer Risk Prediction. Radiology 2024;310:e232780. [Crossref] [PubMed]
- Venkatesan P. Largest trial of AI in breast cancer screening launched. Lancet Oncol 2025;26:285. [Crossref] [PubMed]
- Lång K, Josefsson V, Larsson AM, et al. Artificial intelligence-supported screen reading versus standard double reading in the Mammography Screening with Artificial Intelligence trial (MASAI): a clinical safety analysis of a randomised, controlled, non-inferiority, single-blinded, screening accuracy study. Lancet Oncol 2023;24:936-44. [Crossref] [PubMed]
- Neto PC, Montezuma D, Oliveira SP, et al. An interpretable machine learning system for colorectal cancer diagnosis from pathology slides. NPJ Precis Oncol 2024;8:56. [Crossref] [PubMed]
- Udrea A, Mitra GD, Costea D, et al. Accuracy of a smartphone application for triage of skin lesions based on machine learning algorithms. J Eur Acad Dermatol Venereol 2020;34:648-55. [Crossref] [PubMed]
- Mason F, Pandey AC, Gadaleta M, et al. AI-enhanced reconstruction of the 12-lead electrocardiogram via 3-leads with accurate clinical assessment. NPJ Digit Med 2024;7:201. [Crossref] [PubMed]
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv 2022;8:eabq6147. [Crossref] [PubMed]
- Markus AF, Kors JA, Rijnbeek PR. The role of explainability in creating trustworthy artificial intelligence for health care: A comprehensive survey of the terminology, design choices, and evaluation strategies. J Biomed Inform 2021;113:103655. [Crossref] [PubMed]
- Moy S, Irannejad M, Manning SJ, et al. Patient Perspectives on the Use of Artificial Intelligence in Health Care: A Scoping Review. J Patient Cent Res Rev 2024;11:51-62. [Crossref] [PubMed]
- Petersson L, Larsson I, Nygren JM, et al. Challenges to implementing artificial intelligence in healthcare: a qualitative interview study with healthcare leaders in Sweden. BMC Health Serv Res 2022;22:850. [Crossref] [PubMed]
- Ahmed MI, Spooner B, Isherwood J, et al. A Systematic Review of the Barriers to the Implementation of Artificial Intelligence in Healthcare. Cureus 2023;15:e46454. [Crossref] [PubMed]
- Warraich HJ, Tazbaz T, Califf RM. FDA Perspective on the Regulation of Artificial Intelligence in Health Care and Biomedicine. JAMA 2025;333:241-7. [Crossref] [PubMed]
- Castro DC, Walker I, Glocker B. Causality matters in medical imaging. Nat Commun 2020;11:3673. [Crossref] [PubMed]
- Richens JG, Lee CM, Johri S. Improving the accuracy of medical diagnosis with causal machine learning. Nat Commun 2020;11:3923. [Crossref] [PubMed]
- Bach JF. Causality in medicine. C R Biol 2019;342:55-7. [Crossref] [PubMed]
- Van den Brink N, Holbrechts B, Brand PLP, et al. Role of intuitive knowledge in the diagnostic reasoning of hospital specialists: a focus group study. BMJ Open 2019;9:e022724. [Crossref] [PubMed]
Cite this article as: Sharma RM. Artificial intelligence in medical image analysis and molecular diagnostics: recent advances and applications. J Med Artif Intell 2025;8:53.


