Sleep’s depth detection using electroencephalogram signal processing and neural network classification
Introduction
The electrophysiological signals such as electromyogram (EMG), electrocardiogram (ECG), electro-oculogram (EOG) and electroencephalogram (EEG) are very important to diagnose many human body organs, the EMG is related to the muscle diagnosis, the ECG gives an idea about the heart rate, and all the related parameters of the cardiovascular system if it is running well or not. The EOG represents the electrical activity of the eye blinking and finally the EEG represents the electrical activity of the brain. Each signal must be recorded from the proper region over the human body. And each signal has its own range in terms of frequency and amplitude.
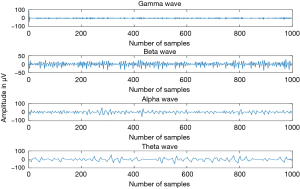
The EEG signal’s frequency is localized between 0.1 and 100 Hz, sometimes we record some frequencies beyond this range, actually it depends on what kind of details the doctor would like to visualize. In this paper our target is to detect the sleep stages; so, the frequency domain 0.1–100 Hz is sufficient to deal with this duty. Because the first task that must be done before the stage detection or classification is, the feature extraction. So, what kind of features will be extracted? And what is the efficient method to deal with that? In the EEG signal processing, it is mandatory to extract the different waves from the EEG time series. There are many waves called respectively alpha (α), theta (θ), beta (β), delta (δ), and gamma (γ). And each wave has its own frequency range (Table 1) and amplitude range as well (1).
Table 1
| Brain waves | Delta (δ) | Thêta (θ) | Alpha (α) | Beta (β) | Gamma (γ) |
|---|---|---|---|---|---|
| Frequency range (Hz) | 0.5–4 | 4–8 | 8–12 | 12–35 | 35–100 |
And after the extraction of all waves, we determine their actual frequency ranges, their amplitude and their power spectrum if we need that.
In order to extract the brain waves (Figure 1), there are many methods, and according to one investigation that we have done in one of our papers (2), the most efficient method is the wavelet transform.
And for sure before all the processing steps, we must perform the EEG signal cleaning in order to remove all the artifacts and we keep only the useful part of the signal. All biosignals and especially the brain signals are basically full of noise. We can classify them into two categories the first one is related to the patient or subject; like the baseline, EMG interference, and some other noises that come from the human body. The second one is related to the recording device such as the power supply interference and the additive white Gaussian noise. In order to remove all the useless components, in fact, there is a plenty of cleaning methods. The most efficient we experienced is the wavelet.
And after the cleaning and the features extraction we will move to the real-time classification of the clean EEG signals. Our aim in this paper is to classify in real-time the EEG signals. In other words, we will detect in real-time in which stage is the patient or the subject. Let’s first have an overview on the sleep stages and the progress of the brain waves during the sleep.
The American Academy of Sleep Medicine (AASM) and Rechtschaffen and Kales (R & K), human professionals find out about the specific time sequence files and assign every time phase to a sleep stage in accordance to reference nomenclature. In this study, we used rapid eye movement (REM) and non-rapid eye movement (NREM) as two usual phases of human sleep. But we focus only on the NREM sleep stages, from 1 to 4. The four sleep stages are very directly dependent on the EEG signal components, hence the variation of the frequency and the magnitude as well. To carry out our demonstration, we must put a number of electrodes on the skull of our subject, during the night or during his/her rest in the daytime.
- Stage 1: in fact, this is the first step in the slight sleep phase, it’s known as a switching stage from wakefulness to sleep when the muscles begin to relax, it takes up to 5 minutes, the EEG signal evolution in this range of time present a low voltage of alpha and theta waves. But this stage appears from 4 to 5 times during the whole sleep time.
- Stage 2: is the “baseline” of sleep and it is called also “light sleep” because it is characterized mainly by the sleep spindles and the K-complexes, of course in this stage there is no eye movements and the brain waves significantly slow down.
- The complete k-complexes ought to final for a minimal of 0.5 sec (3). Alternatively, excessive voltage delta waves might also contain up to 20 proportions of Stage two epochs. The Complete sleep period might also consist of 45–55 shares of Stage two.
- Stage 3: this stage is also called deep sleep and it is referred to a duration all through which at least 20 proportion and no longer greater than 50 shares of the sleep. It consists of EEG indicators with frequencies of 2 Hz or smaller and amplitudes of extra than 75 µV (delta waves). This stage usually appears in the first 30% of the sleep time by a frequency of 4 to 6 during this last.
- Stage 4: this stage is called “very deep sleep” it is a bit similar to Stage 3, but delta wave becomes dominant this stage appears between 12 and 15 times of the whole sleep episode.
And the last big phase of sleep is the REM, which represents 20% to 25% of the normal sleep night, this stage covers all the strange behaviors of people during the sleep night including dreams, nightmare, etc. in this period of sleep the brainwaves (EEG signal) slowdown in terms of voltage.
The sleep stages study using EEG signal analysis is full of challenging tasks, there were other methods of sleep detection and using many other biosignals like the EOG, EMG, etc. but for the application in daily life if we use more signals inevitably, we must use more sensors and more devices, moreover, our solution will be embarrassing for the subject. The fact is that our solution does not concern only people with abnormalities it will be used also for daily life monitoring.
The sleep EEG signal (4) is strongly nonstationary for this reason, the wavelet transformation remains the most efficient for sleep EEG signal processing and evaluation. Wavelet radically change has been used for sleep staging (5) and alertness degree detection (6,7). Rechtschaffen et al. (8) in contrast the overall performance of 5 linear and quadratic classifiers, k nearest neighbors, Parzen kernels and neural networks in computerized sleep stages classification. Therefore, right here we deployed wavelet radically change and artificial neural networks (ANNs). the use of the EEG alerts in this work is to distinguish between the different states of our subjects, Awake, Stage1 + REM, Stage 2 and Sluggish Wave Sleep.
EEG signal waveform during sleep
The EEG signal changes in terms of frequency and amplitude as well, when the patient or the subject moves from one sleep stage to another (Figure 2). the EEG signal of an awake person is more reach of frequency in comparation to the sleep person whatever its stage.
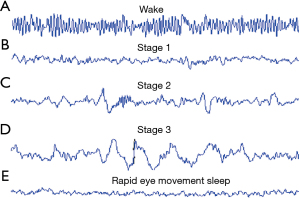
When the subject is awake, his EEG waveform is reach of frequency because the brain is significantly active as illustrated in Figure 2A.
When the subject is in stage 1 of sleep, his EEG waveform starts to decrease in terms of frequency, as illustrated in Figure 2B.
When the subject is in stage 2 of sleep, his EEG waveform continues to slow down in terms of frequency, as illustrated in Figure 2C.
When the subject is in stage 3 of sleep, his EEG waveform continues to slow down in terms of frequency and amplitude, as illustrated in Figure 2D.
When the subject is in the REM sleep, his EEG waveform start to get fast and the frequency increases slightly, as illustrated in Figure 2E.
EEG signal processing scenarios
Signal characterization based on Frequency ranges
The EEG signal describes the electrical activity of the brain. But it doesn’t give an idea about the brain but the whole body. Especially the mood, the sleep state and many phenomena related to the human being state. Many brain disturbances can be featured by visual checking of the EEG signals. The experts in neurology and neuroscience such as doctors and physiologists, can distinguish easily if there are some abnormalities in the brain rhythm or not, due to their experience in the field. In all cases, there are normal ranges in terms of frequency and likewise for the magnitude. For example, when the patient or the subject moves from a mental state to another.
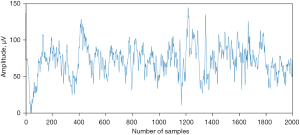
The EEG signal has an amplitude in the order of µvolts with a frequency that can reach 300 Hz. The recorder signal must be amplified before all signal conditioning stages including; digital-analog conversion, filtering, thresholding, etc. in order to make it useful for doctors as interpreters and for researchers since it is their field of study because the raw EEG signal is full of artifacts as illustrated in Figure 3, the patient related ones and external ones (9).
The neural activity of the human brain starts between the 17th and 23rd weeks of prenatal development. It is believed that from this early stage and throughout life electrical signals generated by the brain represent not only the brain function but also the status of the whole body. This assumption plays an important role to motivate researchers for applying advanced digital signal processing methods to the EEG signals measured from the human brain (10).
The EEG signals can be used to diagnose several brain disorders like epilepsy, apnea, etc. one EEG signal that we record from one electrode consists of many waves. They are distinguished by their frequencies and their amplitude. They are called respectively alpha (α), theta (θ), beta (β), delta (ẟ), and gamma (γ). Berger mentioned (α) and (β) in 1929, after seven years Walter announced the delta (ẟ) rhythm to represent all frequencies less than alpha (α) and also, he called the range 4–7.5 Hz as theta (θ) wave. Then after that Jasper and Andrews used the symbol (γ) to describe waves that frequencies above 30 Hz (11).
Pre-processing of EEG signal
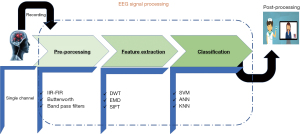
Actually, it is very challenging to detect the noise part of the EEG signal, because it includes many artifacts, usually there are two kinds of artifacts or noises, the first category is related to the subject like the motions, the breathing signals, EMG, ECG, etc. the second type of noise depends on the recording device the famous nose the power supply frequency. For this purpose, it is strongly recommended to use batteries to empower the recording devices. It is very important to have a clean signal in order to extract the features and make decisions accordingly (11-15). As our aim in this paper is sleep detection using the EEG signal, according to several previous works and papers; we make the pre-processing before extracting the features. In this regard, there is a plenty of methods of EEG signal pre-processing, too many researchers use the frequency selective filtering such as finite impulse response (FIR) or infinite impulse response (IIR) also the other selective digital filters (low pass, high pass, band-pass and band-stop filters) have been used to remove the both mentioned artifacts from the EEG signal. And some others use adaptive filtering methods like discrete wavelet transform (DWT) because it is more adaptive for non-stationary signals. In biosignals processing and especially electrophysiological signal, the filtering task is a bit difficult, because of the required types of filters. What is strongly important is the linear phase function in order to perform the pre-processing efficiently, for this purpose, we use Butterworth and elliptic IIR filters (Figure 4).
Wavelet transform
The DWT is a time frequency method of signal processing, according to many data scientists the DWT is the most suitable method for non-stationary signals, thus it is appropriate for EEG signal processing. The strength of this method lies in the time-frequency analysis (16-19). Thus, in this paper, we use the WT for the features extraction and as our features are mainly the brainwaves. Moreover, the features extraction is the most sensitive and critical step in biosignals processing. Dornhege et al. (20-22), because the classification is based on the preprocessed data, so this last must be clean and integral that we must keep only the useful part of the EEG signal and eliminate all the other components. That is why we made decision that the wavelet packet transform (WPT) is the effective method for this task.
With
ψ(t): The wavelet function;
φ(t): The companion function;
CN,K: The approximation coefficients at level N;
dj,k: The detail coefficients or wavelet coefficients.
The function above describes only the standard wavelet function but many studies proved that we must have a wavelet orthonormal basis because always that function must satisfy the tradeoff between the localization of time and frequency. The most useful theorem is that one of Parseval as described by the equation below.
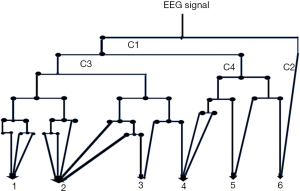
In our study, we used a WPT with 7 levels in order to extract the brainwaves (Figure 5), Daubechies order two (db2) wavelets radically change used to be utilized to 30-second epochs of EEG signal (23). And we selected manually the frequency ranges according as provided in Table 2.
Table 2
| Brainwave | Delta | Theta | Alpha | Spindle | Beta |
|---|---|---|---|---|---|
| Frequency range (Hz) | 0.39–3.13 | 3.13–8.46 | 8.46–10.93 | 10.93–15.63 | 15.63–21.88 |
WPT and selected sub bands
1. {0.39–<3.13 Hz}, Delta, Wavelet coefficient =[C38, C30, C31, C32]= S1
2. {3.13–<8.46 Hz}, Theta, Wavelet coefficient =[C33, C34, C22, C23, C35]=S
3. {8.46–<10.93 Hz}, Alpha, Wavelet coefficient =[C36, C25]= S3
4. {10.93–<15.63 Hz}, Spindle, Wavelet coefficient =[C26, C27, C28]= S4
5. {15.63–<21.88 Hz}, Betal, Wavelet coefficient =[C16, C17]= S5
6. {21.88–<37.50 Hz}, Beta 2, Wavelet coefficient =[C18, C5]= S6
Feature extraction
In order to extract the features by using the time frequency analysis, we performed that procedure in four stages as follow:
- Calculate the energy (E1 to E6) of the wavelet packet (WP) coefficients using Parseval’s equation for all the 6 bands one by one.
- Calculate the total energy E7.
- Calculate the Ratio of energy values of (E8, E9, E10).
- Calculate the Mean absolute values of the coefficients in every sub-band.
- Calculate Standard deviation of all the coefficients in each sub band.
Classification of neural networks
In fact, the automatic sleep depth detection can be done using several methods. Nakayama et al. (24) already performed a comparison of 5 linear and quadratic classifiers, k-nearest neighbors, Parzen kernels and neural networks. According to that deep comparative study. There are also other methods using a single EEG lead or electrode. Mizuno et al. (25-27). But it was proven that the most efficient method is to use multichannel EEG signal with the ANNs classification.
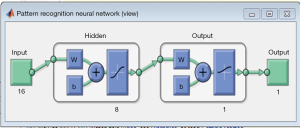
The back propagation algorithm is the workhorse of learning in neural networks. It is a common method of training ANNs used in conjunction with an optimization method. Back propagation requires a known, desired output for each input value, neural net. Actually, back propagation1, 2, 3 is the coaching or getting to know algorithm as an alternative to the community itself. These are referred to Feed-Forward Networks or every now and then multi-layer perceptron’s (MLPs). The community operates in precisely the equal way as the others we’ve seen. A Back Propagation community learns by using example. You provide the algorithm examples of what you prefer the community to do and its adjustments the network’s weights so that (Figure 6), when education is finished, it will supply you with the required output for a specific input. Back Propagation networks are best for easy pattern recognition and mapping tasks 4.
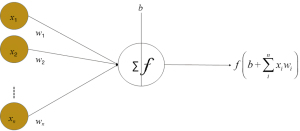
As simply mentioned, to instruct the community you want to supply its examples of what you want. the output you favor (called the Target) for a specific input (28-31).
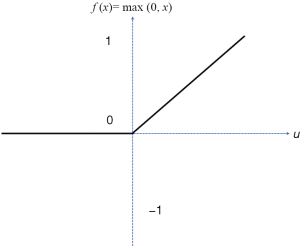
f is the activation function and there are several types of such function (Figure 7). According to many previous papers; when we are dealing with deep learning issues, the recommended one is the rectified linear unit (RLU) (32-34).
The RLU is the most ordinarily utilized initiation work in deep learning models. The capacity returns 0 in the event that it gets any bad info, however for any certain worth x it returns that worth back. So, it tends to be composed as follow (35): f (x)= max (0, x).
- First practice the inputs to the community and work out the output—understand this preliminary output ought to be anything, as the preliminary weights had been random numbers.
- Next work out the error for neuron B. The error is What you prefer, What you clearly get.
The solitary association learning in a back engendering calculation (Figure 8). The association we’re engaged with is between neuron (a hidden layer neuron) and neuron B (a yield neuron) and has the WAB. The format moreover recommends some other association, between neurons A and C, anyway we’ll get back to that later in this paper.
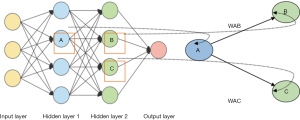
Back engendering has a few issues related to it (36). Maybe the charming perceived is called “Neighborhood Minima”. This happens because of the reality the calculation persistently changes the loads so as to reason the mistake to fall. If so, the calculation will “stalls out” and the blunder will now not lessen further. There are different alternatives to this issue. One is extremely simple and that is to reset the loads to remarkable irregular numbers and endeavor instructing again (this can also clear up various issues) (37-39). Another answer is to add “force” to the weight change. This expertise that the weight substitutes this new delivery depends upon now not just on the current day blunder, anyway furthermore on going before changes.
W+ = W + Current trade + (Change on going before new delivery × steady) under 1.
There are other lesser referred to issues with Back spread too. These will in general show themselves as the organization gets bigger, however many can be overwhelmed by reinitializing the loads to various beginning qualities (40-44).
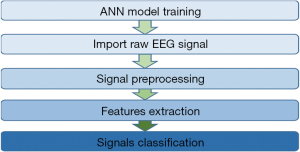
The raw EEG signal is collected and it is preprocessed using wavelet transform. Then the entropy-based features are extracted and the features are fed to the trained neural network. The neural network will perform the classification (Figure 9) and the classified result is displayed in the graphic user interface (GUI).
Results and discussions
The highlights were extracted from 30-second fragments of the PzOz channel EEG signal. Prior to grouping, it was consequently essential that every one of the highlights is scaled to such an extent that the weighting of any element does not assume a more significant part than some others.
After performing the training of our model (Figure 10) and using PhysioNet database Sleep EEG signals as our inputs, the ANN algorithm gave good results in terms of classification, it could distinguish different sleep degrees, especially the four targets Awake, stage 1, stage 2 and finally the slow wave sleep (SWS) which helps the medical staff to analyze the sleep state of each subject (Table 3).
Table 3
| Sleep state | Awake | Stage 1 | Stage 2 | SWS |
|---|---|---|---|---|
| Sensitivity, % | 80.7 | 84.3 | 86.1 | 82.7 |
| Accuracy, % | 97.2 | 90.9 | 88.5 | 96.4 |
SWS, slow wave sleep.
Irrespective of all the study that was performed in this paper to detect the depth of sleep using the EEG signal single channel and the ANN, many other studies and works can be done to enhance the sleep depth detection. Another important point that we must consider about is that the EEG analysis is not used only for the brain diagnosis but also it plays an important role in the daily life monitoring as it is a non-invasive procedure.
Conclusions
In our paper, we started by extracting features using WPT, and after that we performed the classification by using the deep learning. The real-time sleep detection using EEG signal is the most effective method for two reasons. The first one is noninvasive procedure. And the second reason is very precise one, cause all the human body behaviors including sleep are initiated by the brain. Even though the EEG signal recording has some drawbacks, since the EEG headset or electrodes are annoying little bit.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jmai.amegroups.com/article/view/10.21037/jmai-22-32/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kemp B, Zwinderman AH, Tuk B, et al. Analysis of a sleep-dependent neuronal feedback loop: the slow-wave microcontinuity of the EEG. IEEE Trans Biomed Eng 2000;47:1185-94. [Crossref] [PubMed]
- Touil M, Bahatti L, Elmagri A, et al. EEG signal cleaning for drowsiness detection. 2020 International Conference on Electrical and Information Technologies (ICEIT), 2020:1-5.
- Shepard JW 2nd. editor. Atlas of Sleep Medicine. Futura Publishing Company, 1991.
- Estrada E, Nazeran H, Barragan J, et al. EOG and EMG: two important switches in automatic sleep stage classification. Conf Proc IEEE Eng Med Biol Soc 2006;2006:2458-61. [Crossref] [PubMed]
- Oropesa E, Cycon HL, Jobert M. Sleep Stage Classification using Wavelet Transform and Neural Network. International Computer Science Institute, 1999.
- Kiymik MK, Akin M, Subasi A. Automatic recognition of alertness level by using wavelet transform and artificial neural network. J Neurosci Methods 2004;139:231-40. [Crossref] [PubMed]
- Becq G, Charbonnier S, Chapotot F, et al. Comparison between five classifiers for automatic scoring of human sleep recordings. Classification and Clustering for Knowledge Discovery. Springer, 2005:113-27.
- Rechtschaffen A. Kales A. editors. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Public Health Service, Washington DC: US Government Printing Office, 1968.
- Fraiwan L, Lweesy K, Khasawneh N, et al. Time frequency analysis for automated sleep stage identification in fullterm and preterm neonates. J Med Syst 2011;35:693-702. [Crossref] [PubMed]
- Khalighi S, Sousa T, Pires G, et al. Automatic sleep staging: A computer assisted approach for optimal combination of features and polysomnographic channels. Expert Syst Appl 2013;40:7046-59. [Crossref]
- Alickovic E, Subasi A. Ensemble SVM Method for Automatic Sleep Stage Classification. IEEE Trans Instrum Meas 2018;67:1258-65. [Crossref]
- Chien YR, Wu CH, Tsao HW. Automatic Sleep-Arousal Detection with Single-Lead EEG Using Stacking Ensemble Learning. Sensors (Basel) 2021;21:6049. [Crossref] [PubMed]
- Gamboa P, Varandas R, Rodrigues J, et al. Attention Classification Based on Biosignals during Standard Cognitive Tasks for Occupational Domains. Computers 2022;11:49. [Crossref]
- Carli F, Ferrillo F, Gabarra M, et al. Wavelet analysis and adaptive classification in a system for automatic sleep staging. In: Analysis of the electrical activity of the brain, 1997:253-59.
- Stanus E, Lacroix B, Kerkhofs M, et al. Automated sleep scoring: a comparative reliability study of two algorithms. Electroencephalogr Clin Neurophysiol 1987;66:448-56. [Crossref] [PubMed]
- Thakor NV, Tong S. Advances in quantitative electroencephalogram analysis methods. Annu Rev Biomed Eng 2004;6:453-95. [Crossref] [PubMed]
- Burros C, Goliath R, Guo H. Introduction to wavelets and wavelet transforms. Prentice Hall Pub, 1998.
- Webster JG. Medical Instrumentation, Application and Design. 3rd Edition, Wiley, 1998.
- Efron B, Tibshirani RJ. An introduction to the bootstrap. CRC Press, 1994.
- Dornhege G, Millán J R, Hinterberger T, et al. Toward brain-computer interfacing. Cambridge, MA: MIT Press, 2007.
- Berger TW, Chapin JK, Gerhardt GA, et al. Brain-Computer Interfaces: An international assessment of research and development trends. Dordrecht: Springer, 2008.
- Hortal E, Úbeda A, Iáñez E, et al. Online classification of two mental tasks using a SVM-based BCI system. 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER), 2013:1307-10.
- Nasehi S, Pourghassem H. Mental task classification based on HMM and BPNN. 2013 International Conference on Communication Systems and Network Technologies. IEEE, 2013:210-4.
- Nakayama K, Inagaki K. A brain computer interface based on neural network with efficient pre-processing. 2006 International Symposium on Intelligent Signal Processing and Communications. IEEE, 2006:673-6.
- Anderson CW, Bratman JA. Translating thoughts in to actions by finding patterns in brain wave. In: Proceedings of the Fourteenth Yale Workshop on Adaptive and Learning Systems, Yale University New Haven CT; 2008:1-6.
- Mizuno Y, Mabuchi H. Clustering of EEG data using maximum entropy method and LVQ. Int J Comput 2010;4:193-200.
- Hosni SM, Gadallah ME, Bahgat SF, et al. Classification of EEG signals using different feature extraction techniques for mental-task BCI. 2007 International Conference on Computer Engineering & Systems. IEEE, 2007:220-6.
- Tolić M, Jović F. Classification of wavelet transformed EEG signals with neural network for imagined mental and motor tasks. Kinesiology 2013;45:130-8.
- Keirn ZA, Aunon JI. A new mode of communication between man and his surroundings. IEEE Trans Biomed Eng 1990;37:1209-14. [Crossref] [PubMed]
- Huan NJ, Palaniappan R. Neural network classification of autoregressive features from electroencephalogram signals for brain-computer interface design. J Neural Eng 2004;1:142-50. [Crossref] [PubMed]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 1958;10:370-5.
- Shedeed HA, Issa MF, El-Sayed SM. Brain EEG signal processing for controlling a robotic arm. 2013 8th International Conference on Computer Engineering & Systems (ICCES). IEEE, 2013:152-7.
- Yang G, Nakayama K, Hirano A. A dual-class voting mechanism for brain computer interface based on wavelet packet and support vector machine. IEEE Conference Anthology. IEEE, 2013:1-5.
- Shedeed HA, Issa MF. Brain-EEG signal classification based on data normalization for controlling a robotic arm. Int J Tomogr Simul 2016;29:72-85.
- Fraiwan L, Lweesy K, Khasawneh N, et al. Automated sleep stage identification system based on time-frequency analysis of a single EEG channel and random forest classifier. Comput Methods Programs Biomed 2012;108:10-9. [Crossref] [PubMed]
- Vatankhah M, Akbarzadeh-T MR, Moghimi A. An intelligent system for diagnosing sleep stages using wavelet coefficients. The 2010 International Joint Conference on Neural Networks (IJCNN). IEEE, 2010:1-5.
- Wu HT, Talmon R, Lo YL. Assess sleep stage by modern signal processing techniques. IEEE Trans Biomed Eng 2015;62:1159-68. [Crossref] [PubMed]
- Kottaimalai R, Rajasekaran MP, Selvam V, et al. EEG signal classification using principal component analysis with neural network in brain computer interface applications. 2013 IEEE international conference on emerging trends in computing, communication and nanotechnology (ICECCN). IEEE, 2013:227-31.
- Chang CC, Lin CJ. LIBSVM. A library for support vector machines. ACM Trans Intell Syst Technol 2011;21-7.
- Palaniappan R, Paramesran R, Nishida S, et al. A new brain-computer interface design using fuzzy ARTMAP. IEEE Trans Neural Syst Rehabil Eng 2002;10:140-8. [Crossref] [PubMed]
- Hassanien AE, Azar AA. Brain-computer interfaces: trends and applications. Switzerland: Springer; 2015.
- Ronzhina M, Janoušek O, Kolářová J, et al. Sleep scoring using artificial neural networks. Sleep Med Rev 2012;16:251-63. [Crossref] [PubMed]
- Chien YR. Wavelet packet transform-based anti-jamming scheme with new threshold selection algorithm for GPS receivers. Journal of the Chinese Institute of Engineers 2018;41:181-5. [Crossref]
- Harender, Sharma RK. EEG signal denoising based on wavelet transform. 2017 International conference of Electronics, Communication and Aerospace Technology (ICECA). IEEE, 2017:758-61.
Cite this article as: Touil M, Bahatti L, El Magri A. Sleep’s depth detection using electroencephalogram signal processing and neural network classification. J Med Artif Intell 2022;5:9.