
Navigating the horizon of opportunity: a comprehensive review of artificial intelligence applications in cancer care—insights from the 2024 landscape, a narrative review
Introduction
Over the course of the last 50 years, the field of cancer care has undergone a transformative journey, achieving unprecedented success and consistently evolving to enhance patient outcomes. This remarkable progress is evident in the continuous stream of innovations, discoveries, and advancements that have reshaped the way we understand, diagnose, and treat various forms of cancer (1).
These successes have gone hand in hand with increasing complexity of modern cancer care. The continuous advancements have been achieved through a high level of effort and an intricate web of human input. The complexity arises from the integration of cutting-edge technologies, personalised treatment approaches, a continuously updating number of treatment options and scheduling, and the multifaceted nature of addressing the physical, emotional, and psychosocial aspects of cancer. This cancer workforce is expected to deliver complex cancer care for an ever-increasing number of people, who are invariably now living much longer than in previous decades.
However, amidst this backdrop, the emergence of artificial intelligence (AI), particularly machine learning and foundational models has opened up a new horizon of opportunities, novel applications and possibilities in cancer medicine. Numerous technological solutions are in development, and could enable more rapid knowledge dissemination, analysis of vast datasets, better optimise clinician time and drive enhancing patient outcomes (2).
In spite of the complexity of cancer care, at its core, health systems must bring together a patient and clinician and deliver four crucial steps that provide effective cancer care. Firstly, health systems must identify patients, through either symptomatic or screening approaches. Secondly, systems must develop the optimal management plan for the patient to gain the greatest benefit. Thirdly, systems must be able to deliver effective care that is tailored to healthcare resources and to individual patient needs. Finally, there should be a clear signposting process for a patient who has completed their cancer care journey.
Our 2024 landscape review covers each of these four steps in cancer care and describes how each of these steps could be accelerated or are being impacted by AI. This includes diagnostics and imaging to help with patient identification, genomics, AI driven drug discovery and development and clinical trial optimisation for treatments to help with developing the optimal management plan, foundational models in ensuring that effective care is delivered and there is a clear signposting process for patients (Figure 1).
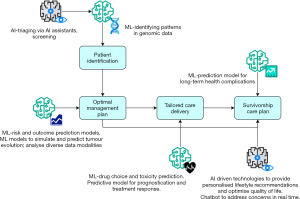
However, it must be noted that the integration of machine learning into the clinical cancer environment is at its early stages, leaving ample room for further advancements. Several factors contribute to this, such as the absence of high-quality and varied datasets, the lack of comprehension for inter-tumour heterogeneity, and the current challenge of keeping pace with the evolving features of cancer throughout the treatment duration. This article explores current and future applications of machine and deep learning in oncology and discusses the ethical implications of integration of AI software tools into clinical practice and how these may be overcome to ensure that the next 50 years of cancer care could be as pivotal as the last. We present this article in accordance with the Narrative Review reporting checklist (available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-38/rc).
Methods
A literature search for peer-reviewed publications in three databases including PubMed, MEDLINE and Google Scholar was performed, looking at articles published in the last 5 years (Table 1). Search terms used included “artificial intelligence”, “machine learning”, “convolutional neural networks”, “deep learning”, “AI in cancer”, “AI in imaging”, “AI in prognostication” and “AI powered technologies”. Non-English and non-peer-reviewed articles were excluded from this analysis as well as other papers deemed to be irrelevant to the scope of our paper. This was done independently by reviewers Y.A.H. and F.Z.A.H., and were discussed with remaining author L.Y.W.L. to reach a consensus regarding what articles to include.
Table 1
| Items | Specification |
|---|---|
| Date of search | January 2, 2024 |
| Databases and other sources searched | PubMed, MEDLINE, Google Scholar |
| Search terms used | “Artificial intelligence”, “deep learning”, “AI in cancer”, “AI in imaging”, “machine learning”, “convolutional neural networks”, “AI powered technologies”, “AI in prognostication” |
| Timeframe | 2018 to 2023 |
| Exclusion criteria | Non-English and non-peer-reviewed articles were excluded |
| Selection process | Reviewers Y.A.H. and F.Z.A.H. conducted the selection independently, and reviewed this with L.Y.W.L. for complete consensus |
AI, artificial intelligence.
Identify
Triaging patients
Early and accurate identification of patients with cancer begins with effective triage which can play a significant role in reducing unnecessary referrals, reducing waiting times for diagnosis and treatment and overall improving cancer outcomes for patients. Since the inception of the 2-week wait (2WW) referral pathway, which involves a general practitioner referring a patient with a suspected cancer diagnosis to be seen within 14 days, there has been an increase in referrals by approximately 10% every year, with 2.8 million referrals in 2022 (3). Integrating AI into patient triage systems will allow for a clinically impactful diagnostic pathway that is more robust than clinicians simply using certain criteria to refer patients, and improve resource allocation. An example is PinPoint, a machine learning driven blood test for cancer that has been designed to optimise the 2WW pathway by indicating the likelihood of a patient having cancer, thereby enabling only those deemed high risk to have further investigations (4). PinPoint has already been deployed across five regions in the UK as a clinical decision support tool, but widespread use of such a platform is necessary to reduce national health inequalities and ensure better outcomes for all patients with cancer.
The use of AI chat boxes as a tool to support clinicians with decision making has great potential particularly in the emergency setting where clinicians are under time pressure and patient outcomes are often very time-sensitive. Gebrael et al. explored the use of ChatGPT 4.0, an advanced AI system, in improving triage decisions for emergency room patients with metastatic prostate cancer. The findings demonstrated ChatGPT’s high sensitivity in determining patient admission, accurate diagnosis and provision of additional treatment recommendations (5). These results highlight the potential of machine learning to enhance patient triage, improve efficiency, and elevate the quality of care in emergency services for individuals with cancer (5). Incorporating patient reported outcomes and socioeconomic factors with a machine learning model to analyse symptom severity, for example, would inform triage decisions further and prioritise patients most likely to deteriorate or those with a poorer prognosis (6). Furthermore, this would help with reducing longer wait times, unnecessary admissions and resource wastage which can negatively impact patient quality of care.
Diagnostics: imaging
Improving cancer care and prognosis necessitates the early identification of symptomatic patients and screening those at high risk of cancers. Medical imaging plays a significant role in these early stages and is largely performed by clinical radiologists to confirm the presence or absence of disease, define tumour margins, monitor response to treatment and identify signs of relapse. Integration of AI in diagnostic cancer medicine has shown great promise in revolutionising early cancer diagnosis, increasing diagnostic accuracy, reducing workload for radiologists and enhancing risk stratification (7). This is particularly important given the disruption coronavirus disease 2019 (COVID-19) pandemic has caused to screening programmes, resulting in diagnostic delays and poorer outcomes for patients with cancer (8).
Radiomics is a quantitative approach to medical imaging utilising machine learning which focuses on mathematical analysis of these images to provide clinical decision support and ultimately facilitate the provision of precision medicine (9). Analysis of image texture, volume, density, and vasculature can provide useful information on the tumour microenvironment and therefore generate predictive biomarkers, normally only available through molecular tumour profiling tests. Due to their invasive nature, high costs and time limitations, incorporating these tests into routine clinical care is often difficult; AI will be instrumental in overcoming these challenges and increasing the efficiency and diagnostic and prognostic accuracy of the imaging stage. This can aid the stratification of high-risk patients, early detection of diseased patients and facilitate timely specialist intervention and guide management based. This is particularly important for cancers which are difficult to detect in the early stages due to their non-specific symptoms such as lung and ovarian cancer (10). Report increased staging accuracy using a mathematical software called TEXLab to determine the aggressiveness of ovarian tumours based on preoperative computed tomography (CT) scans (10). This resulted in 4 times more accurate prognostication, specifically in predicting mortality from ovarian cancer for each patient, in comparison to prognostic tools used by doctors. This plays a pivotal role in guiding management options and providing treatment most likely to be efficacious, as well as reducing resource wastage by decreasing the chance of offering treatments unlikely to benefit the patient. Furthermore, this also reduces the chance of false positives which is likely to significantly contribute to the stress patients are likely already experiencing.
Given the extensive use of imaging in cancers associated with the highest mortality rates in the UK such as breast, lung and prostate cancer, AI imaging models have the potential of reshaping the diagnostic timeline and treatments for patients with cancer. This is particularly the case with cancers that rely on biopsies to stage and identify the cancer subtype where misdiagnosis or omissions may occur (11). A study by Chen et al. included images from 301 CT scans of lung cancers and through the use of convolutional neural network (CNN), was able to accurately detect lung cancer with 0.82 precision and 0.93 sensitivity (12). Going even further, this model was able to detect subtypes of lung cancer such as small cell carcinoma, adenocarcinoma and squamous cell carcinoma. Additionally, the largest randomised controlled trial investigating the accuracy of AI in breast cancer mammography screening has highlighted the higher recall call rate for further investigations using AI supported by one radiologist as opposed to two radiologists without AI input, leading to 41 more diagnoses of breast cancer (13). This underscores the safety and increased accuracy in using AI within screening programmes, reducing the likelihood of missed diagnosis, poorer prognosis, and reducing screen-reading burden for radiologists. Furthermore, the major advantage of AI technologies is the elimination of inter-reader variability meaning even inexperienced radiologists can use this technology to reach an accurate diagnosis. Given the major differences in treatment between these types of lung cancer, being able to gain such information non-invasively is transformational and can aid in starting treatment early.
Quality assurance in diagnostic imaging is critical for ensuring accurate diagnosis and timely treatments, particularly in the case of conditions like cancer where an early detection can significantly impact outcomes. By integrating AI into the imaging process, it can play a pivotal role in providing feedback on image quality. By analysing images and flagging issues such as inadequate coverage of critical anatomy or poor image quality. AI algorithms can help radiologists and imaging technicians ensure that the images they capture are of sufficient quality for interpretation; this can help reduce the likelihood of missed diagnosis and improve overall patient care. In addition to this, AI can also assist in optimising imaging parameters in real-time to improve image quality, such as adjusting contrast or exposure settings based on the specific characteristics of the patient or the region of interest being imaged. Integrating AI into quality assurance processes for diagnostic imaging holds great promise for enhancing the accuracy and reliability of diagnosis, leading to better patient outcomes.
Diagnostics: genomics
The development and delivery of optimal management plans for patients with cancer relies on the provision of personalised medicine. Next generation sequencing (NGS) has revolutionised cancer medicine by facilitating the achievement of high precision medicine through identification of cancer associated mutations in the genome as well as characterisation of tumour heterogeneity. While significant progress has been made in cancer genomics using NGS, there are limitations due to the high-dimensional and great heterogeneity of datasets within cancer medicine that may be targeted using AI approaches. Given how both AI and genomic medicine are data-driven sciences, there is great scope for integration of AI and genomics to reshape what can be achieved in terms of precision and reliability. Applications of AI in genomic medicine include the discovery of biomarkers to aid in patient stratification, the prediction of gene function and identification of aberrant protein interactions and genetic mutations. The possibility of integrating machine learning, specifically deep learning, and different data-types such as imaging data, genetic mutations and molecular profiling, known as multimodal learning, facilitates more in-depth analysis of multilayered data which is currently a challenge with NGS lacking computational approaches. Before the application of AI, genomic sequences are transformed into binary tables using one-hot encoding and then subjected to one-dimensional convolutional filters. Deep learning offers advantages in multitask learning, where AI simultaneously learns multiple tasks by sharing parts of a model, and in multimodal learning, integrating different data types. This integration aids in analysing large omics datasets and identifying drug-susceptibility genes. For instance, Watson for Genomics identified genomic alterations with clinical effects in 323 patients, surpassing conventional molecular tumours panels (14).
The role of microbiome in cancer is a new area of research; studying the composition and function of the microbiome in and around tumours could shed light into the connections between microbial communities and cancer development which would aid with treatment response. The use of deep learning, more specifically convolutional or deep neural networks, following NGS for the identification of microbial species present, would allow for predicting survival outcomes or the likelihood of disease recurrence for patients making provision for personalised and early treatment.
An emerging strategy for cancer detection utilises deep sequencing for whole blood cancer screening, allowing for the comprehensive analysis of genetic material in a patient’s blood to detect the presence of cancer. Whole blood cancer screening using deep sequencing offers a minimally invasive method for detecting cancer removing the need for invasive tissue biopsies. There are many advantages to do so, including the possibility for the early detection of cancer and the comprehensive profile of the genetic makeup of tumours which would in turn reduce the need for repeated biopsies. Deep sequencing also generates large amounts of genomic data; the integration of machine learning algorithms can enhance the analysis process by identifying patterns and correlations within the complex datasets thus reducing false positives.
Plan
Risk and outcome prediction models
Providing healthcare that is tailored to an individual’s needs as well as healthcare resources partly relies on accurate treatment response prediction models. The use of machine learning and deep-learning assisted cancer prediction models will be instrumental in detecting disease at an earlier stage, thereby improving the scope of intervention and enabling the delivery of more personalised medicine. Yeh et al. demonstrated the use of a deep-learning approach called CNN to predict lung cancer in patients with pre-existing lung conditions within 1 year with excellent performance (15). Their approach relied on data extracted from patients’ medical records, such as existing diagnoses and medications rather than self-reported parameters such as family history and smoking history. This provides an efficient method to digitally screen large populations and identify those at high risk of developing lung cancer in the future, enabling preventative and surveillance measures to optimise patient outcomes. This is especially important considering lung cancer remains one of the leading causes of mortality in the UK and is a highly sensitive condition. The CanPredict model has also shown excellent performance in risk prediction for lung cancer and takes into account self-reported parameters (16). This could play an integral role in the creation of a more efficient targeted lung cancer programme in the UK which is currently being developed, but necessitates the provision of information based on 17 questions patients must answer (16). Additionally, it is limited to those aged between 55–74 years who have previously or currently smoked. Collecting such data and collating it in order to select those suitable for screening is likely to be a time-consuming resource heavy process, but could be overcome by integrating an AI risk prediction model that requires less information without compromising accuracy for these predictions (17,18). However, the ramifications of potential false-positives using such a model must be considered given the high radiation exposure from CT scans.
Epigenetics refers to the study of factors that can result in genetic modification without DNA alteration, and includes processes such as DNA methylation, histone protein modification and non-coding RNA action. The development of NGS and its application to epigenetic analysis has given us a greater understanding of epigenetic factors that may contribute to the development of cancer, and therefore allow for better therapeutic targeting and earlier detection of cancer. However, heterogeneity in epigenetics even within the same cancer type can be high and poses a challenge to identifying the most important factors that determine clinical outcomes for patients (19-21). Implementing AI models to find patterns in the expression of various genes and identify their possible effect on prognosis through the use of multimodal datasets will aid outcome predictions, providing insight into potential therapeutic options. Several studies have demonstrated the use of AI in predicting cancer survival and outcomes (22,23). Pei et al. demonstrated the ability for AI to analyse the epigenetic landscape for adult patients with 24 types of cancers, and detect significant prognostic genes (11). Subsequently, this model was able to differentiate between those with a good or poor prognosis and allow for an estimated survival time. This is of great value in the clinical setting to guide clinicians in selecting the most appropriate treatment option for each patient, and also avoiding unnecessary treatments therefore reducing resource wastage. Continuing to develop AI prediction models will allow clinicians to deliver personalised medicine and maximise the chances of patients commencing treatment most likely to be efficacious for them.
Drug discovery and design
Anti-cancer drug resistance is a growing problem clinicians and patients face, and can limit the treatment options available for patients with cancer preventing the delivery of optimal care. Drug development and design for targeted anticancer therapies is highly advantageous as it offers fewer side effects, drug resistance and is highly efficacious. However, it can be an expensive and lengthy process which is a significant problem given increasing anti-cancer drug resistance. For example, cancers with high genetic heterogeneity exhibit high levels of anti-cancer therapy resistance thus often lack sufficient data for conventional drug discovery methods, prompting the use of machine learning to develop models that leverage transfer learning. Machine learning has the potential to streamline and accelerate drug discovery making the process more efficient and less resource-intensive. This approach aims to generalise insights from data-rich cancer types to address the scarcity of data for genetically heterogeneous cancers, ultimately aiding in developing molecularly aimed treatments. For example, Tong et al. have demonstrated the use of one class vector support machine to predict potential drug targets for liver cancer through computational integration of clinical data, protein-protein interactions and gene profiles (24).
Deploying sophisticated machine learning models capable of seamlessly integrating and analysing diverse data modalities, including genomics, proteomics, and imaging data would allow a holistic approach that offers a comprehensive view of the molecular landscape of cancer, potentially facilitating the discovery of novel drug targets and biomarkers. As highlighted by Tong et al., harnessing data from multiple modalities holds the potential for enhanced AI technology, mitigating the limitations associated with each type of healthcare data through strategic combinations (24).
Tumours undergo dynamic evolution over time, underscoring the importance of capturing this intricate process for effective drug design. One avenue for achieving this is through the development of machine learning models that simulate and predict tumour evolution (25). These models take into account various factors, including genetic mutations, treatment responses, and clonal selection. Such an approach has the potential to guide the design of adaptive treatment strategies and the development of drugs specifically targeting the evolving profiles of tumours (26). One of the most recent uses of mathematical modelling to predict genetic alterations and understand the clinical implications of tumour evolution is observed by Niida et al. (27); they modelled colorectal cancer evolution by genomic analysis and mathematical modelling. This would allow for more precise and robust clinical data to aid with more accurate drug design for dynamically changing tumours.
Treatment
Drug choice
The selection of drug treatments most likely to be efficacious for individual patients is essential for the delivery of personalised care. Supervised machine learning algorithms, which are algorithms trained on a labelled dataset, contribute to the prediction of cancer patients’ responses to specific drugs, informing strategic decisions for optimal treatment. Algorithms grounded in support vector machine (SVM) methodology have proven effective in forecasting individual patient reactions to conventional chemotherapeutic drugs by analysing gene-expression patterns (28). The application of machine learning-derived prediction algorithms, leveraging correlations between molecular profiles across various cancers and their reactions to therapeutic drugs, shows potential for advancing treatment efficacy. By analysing molecular profiles, machine learning algorithms can identify unique characteristics of individual patients’ cancers which would enable the development of personalised treatment strategies that target specific molecular markers associated with cancer.
Recently, there have been advances made involving drug repurposing which is a cost effective strategy that reuses approved drugs for new medical instances. Turabi et al. discuss the current advances in multi omics analysis and genomics that have used drug repurposing to tackle the problem of needing new drugs for cancer cells developing resistance to chemotherapy (29). The integration of machine learning into drug repurposing for cancer allows researchers to systematically analyse vast and complex datasets to identify novel connections between drugs and diseases and accelerate the discovery and choice of effective treatments.
Drug toxicity prediction
The integration of machine learning algorithms for drug toxicity prediction can reduce cases of toxicity associated with anti-cancer drug administration, thereby reducing complications for patients and reducing healthcare resource wastage. With the growing number of toxicity test failures, it has been observed that approximately 90% of drug candidates entering clinical studies experience failure in the initial stages of clinical trials or the drug approval process as conveyed by Cavasotto and Scardino (30). Improving drug toxicity predictions using machine learning would help with the early identification of toxicity complications before having advanced to costly and time consuming clinical trials thus reducing the likelihood of late-stage failures.
Presently, among the highly developed prediction approaches stands Quantitative Structure-Activity Relationships (QSARs), rooted in chemical structural parameters (31). Machine learning models are being employed to enhance conventional QSAR methods (32), employing a combination of algorithms such as random forest and artificial neural networks. The essential step in toxicity predictions lies in the application and comparison of various machine learning techniques to identify the most precise and robust algorithm. Current studies indicate traction towards silico methods, as portrayed by Yang et al. (33), which involve computer-based simulations and modelling. By leveraging QSRA, toxicogenomics, molecular docking and dynamics simulations and ADME-Tox (Absorption, Distribution, Metabolism, Excretion and Toxicity) and integrating them into a machine learning model, researchers can prioritise drug candidates with lower toxicity profiles and thus reduce the risk of adverse effects.
A method that has not been extensively researched or applied in a clinical setting is the use of patient-derived organoids (PDOs) integrated with machine learning. PDOs are three-dimensional cell cultures derived directly from patient tissues capturing the heterogeneity and complexity of the in vivo environment. They retain key features of the original tissue, making them valuable tools for drug toxicity predictions and thus personalised medicine. Monzel et al. have described an interdisciplinary approach to assess the effect of 6-hydroxydopamine (6-OHDA) on dopaminergic neurons in midbrain organoids; a machine learning classifier was applied on the data to predict neurotoxin-induced perturbations (34).
Handover
Clinical trials
Clinical trials play a pivotal role in advancing medical knowledge and treatment options by thoroughly evaluating the safety, efficacy and potential benefits of novel interventions. The incorporation of AI algorithms and machine learning into clinical trial processes holds promise for enhancing patient recruitment, eligibility determination, trial design optimization, and the prediction of trial outcomes. Addressing the escalating issue of premature clinical trial terminations, exemplified in the research conducted by Kavalci et al. (35) is significant. As of 2023, 14.5% of interventional trials documented in the clinicaltrials.gov registry were prematurely terminated, underscoring the magnitude of the problem (36).
Exploring the implementation of a machine learning model to predict clinical trial termination probabilities emerges as a strategic intervention. This approach not only aligns with resource conservation but also has potential economic implications. AI algorithms, adept at analysing electronic health records (EHRs) and patient data, offer a streamlined means of identifying potential clinical trial candidates. Furthermore, machine learning models contribute to the precision of participant selection by predicting eligibility criteria, increasing efficiency over manual screening as reported by Chow et al. (37). Leveraging historical data through AI analysis enables the identification of pertinent biomarkers and the optimisation of inclusion and exclusion criteria. This multifaceted approach facilitates the simulation and modelling of diverse trial scenarios, with an overarching goal of identifying the most efficient and cost-effective designs.
Among the variety of machine learning models, decision trees, Markov models, and neural networks serve as viable baselines. The selection of a particular model is contingent upon factors such as the complexity of relationships within the data and the specific objectives of the clinical trial design (38). This would also help with the automation of matching eligible patients to clinical trials based on medical history, genetic profile and disease characteristics.
Survivorship care plan
The use of AI-driven technologies to signpost and support cancer survivors will no doubt be an invaluable tool in empowering this group of patients and optimising their physical, psychological and functional needs. This aspect of care for cancer patients is often forgotten about and not smoothly integrated into mainstream cancer care, but arguably has the most significant impact on patients as they try to navigate their life after treatment. The psychological and physical burden on some patients following treatment includes depression and anxiety, increased risk of further diseases, cognitive limitations, and often limits one’s ability to maintain the life one had before diagnosis and treatment, reducing the quality of life (39). These challenges that may be faced can be assessed through digital technologies such as apps utilising questionnaires. The information provided can then be assessed by clinicians and offer the opportunity for referral to relevant services at the early stages, before further decline. Digital technologies can also be useful in delivering information more generally on relevant services that can be accessed for further support, and can have a significantly positive impact on the quality of life that cancer survivors report (40). The creation of chatbots leveraging AI, such as ChatGPT, can be further developed to create a platform that is trained specifically in cancer care to address needs and concerns in real-time, where formal medical advice may not be warranted.
Integrating AI in clinical environments would provide timely, personalised support and information which can help patients and their caregivers. AI tools like chatbots can help improve patient engagement and adherence to follow-up care, enhancing their overall health and well-being. AI chatbots can also facilitate better communication between patients and healthcare providers, ensuring that concerns are addressed promptly and efficiently. This increased level of support can lead to higher satisfaction with care, reduced feelings of isolation, and improved mental health outcomes.
Bibault et al. (41) demonstrated the ability of chatbots to personalise conversations with patients with cancer and provide them with information they found useful that they would not have otherwise asked about. Furthermore, the ability to extend information provided by chatbots to family, caregivers and friends regarding cancer is instrumental in overcoming poor health literacy and educational disparities that often render some people less able to access healthcare when needed.
Another avenue for exploration is the use of AI for the prediction of long-term negative outcomes for patients and therefore allow for interventions to be implemented to minimise the impact on cancer survivorship. Through these predictions, clinicians can better shape the follow-up that is provided to individual patients and make it more personalised, allowing these patients to feel better supported and enabling a smoother transition from tertiary care to primary care. Such tools are in current development as reported by Tzelves et al. (42). and if proved to be efficacious and impactful, can be implemented within several healthcare systems to improve patient quality of life and also reduce the burden on healthcare systems. While these applications of AI are likely to revolutionise cancer care for survivors, the risks of patients replying on apps for medical advice must be carefully evaluated, as well as the reliability of information provided through apps or chatbots.
Ethical implication of AI
The use of AI in many domains including cancer research holds great promise but also raises various ethical considerations that must be considered carefully. Challenges in AI model development, such as data standardisation, bias, the need for large datasets and limited validation, can be overcome with concurrent investments. In oncology, early AI tools in EHRs show promise, visualising patient health like a digital photograph (43). However, ethical considerations in AI cancer research, including data privacy, consent, and fairness, demand careful attention. Interdisciplinary collaboration and adherence to ethical guidelines are essential for the responsible progression of AI in the cancer research industry.
Furthermore, the black box nature of AI, which refers to the uncertainty of AI algorithms in how they produce the outcomes they do, poses a challenge in healthcare settings. This is especially relevant to deep-learning mechanisms and neural-network based approaches which heavily rely on multimodal data interpretation. Though there is growing confidence and trust in the integration of AI into healthcare, it is essential to exercise vigilance when interpreting outputs from AI software programmes and using these outputs for clinical decision making. There are a few ways one can increase the interpretability of a machine learning model; the type of architecture used is important as some architectures, including decision trees and rule-based models, are intrinsically more interpretable than others, including deep neural networks (44). The selection of model features can also affect its interpretability, thus having a trade-off between the complexity of the model to solve a specific problem and interpretability is important. The risk of suboptimal management has the potential to compromise patient safety but also incorrectly identify patients that need further intervention for example. Hence, it is essential to underscore the importance of utilising AI triage systems as a clinical decision support tool and avoid heavy reliance on these systems to manage patient care.
One of the most important requirements for AI-driven methods is generalisability along with fairness and transparency as mentioned above. As concluded by Nakayama et al. (45), model generalisability in machine learning is ideal but is likely not feasible because of dataset shifts across place and time. AI models should not perpetuate or magnify existing biases in diagnosis and treatment. In the review by Pagano et al., it is highlighted that limited representation, biased datasets and the lack of bias control assessments are poised to upend successful implementation (46). More diverse, representative and fair datasets are required for generalisable models to be successful.
Moreover, the reproducibility of AI systems is another challenge considering the diversity of healthcare populations, not just nationally but across the globe. Data drift is a phenomenon referring to differences in the data used to train machine learning and deep learning algorithms and the data used in real life settings. For example, disease patterns, patient population and data acquisition can vary. Although drifts in the input distribution to AI systems may not necessarily impact their performance in terms of generalisation and accuracy, it’s important to ensure the development of machine learning models that are capable of spotting data drift at an early stage and implementing effective strategies to recalibrate the AI and adjust its operating point. By addressing these calibration needs, we can ensure the AI remains robust and effective in varied and changing clinical environments.
Discussion
Our review provides a comprehensive overview of the current landscape regarding the integration of AI in various aspects of cancer care, spanning from triaging patients to treatment and survivorship care. Across these domains, we have highlighted several key findings and implications for the future of cancer care.
Within diagnostic imaging, the utilisation of machine learning has been instrumental in facilitating more in-depth analysis of imaging investigations, and has allowed for earlier detection of disease through risk and outcome prediction models. This is particularly important for cancers which are often detected at a very late stage, where the scope of intervention and successful treatment is limited and therefore mortality rates are high. This underscores the importance of also expediting the process of drug design and discovery, which has the potential to increase the treatment options for patients, as well as ensuring efficacy of these treatments is maximised for each patient. Ensuring the increasing problem of anti-cancer drug resistance is addressed is also pivotal and can be achieved through machine learning models which can predict tumour evolution over time. Furthermore, the accurate prediction of drug toxicity and therapeutic range is essential to minimise the adverse reactions of anti-cancer drugs, reducing the burden of these side effects and improving the quality of life during and after treatment.
In the field of genomics, AI offers exciting opportunities for personalised medicine by integrating diverse data types and analysing complex data sets. By leveraging machine learning algorithms, researchers can identify biomarkers, predict treatment responses, and uncover novel therapeutic targets, ultimately improving patient outcomes. Additionally, AI can facilitate the analysis of microbiome data and enable non-invasive cancer screening methods, revolutionising early detection and intervention strategies. To add to this, the optimisation of clinical trial recruitment and outcome prediction is likely to lead to more cost-effective and efficient trials through the refinement of conclusion criteria, anticipation of trial outcomes and early identification of suitable participants. Moreover, a focus on survivorship care is essential, as patients that end their cancer journey often experience long-life effects of treatment that can have a significant impact on quality of life. AI driven survivorship plans can provide valuable resources and support for cancer survivors, addressing their physical, psychological and functional needs.
However, alongside these promising advancements, our review also identified challenges and ethical implications associated with the use of AI with cancer medicine. The black-box nature of AI can make it difficult to interpret machine learning model outcomes, which may not always appear logical or reasonable to clinicians, and can contribute to a lack of trust in integrating such models into clinical care. Additionally, the lack of generalisability of AI models, due to biased data sets and lack of representations, are likely to pose a challenge to the successful implementation of AI models, and hence more representative data sets and bias control assessments are essential to overcome this.
Conclusions
The increased digitalisation of medicine and rapid evolution of AI highlighted the potential of transforming cancer medicine to achieve better patient outcomes. Integration of AI from the beginning of the journey for a patient with cancer will no doubt increase the efficiency of this process, by allowing for earlier detection of diseased patients, improving reliability of imaging and other investigative results, as well as facilitating personalised medicine through accurate prognostication. Furthermore, the use of AI-powered technologies in facilitating the provision of educational resources and support for those who have completed their cancer journey is an exciting prospect that is increasingly more relevant as survival rates for patients with cancer continue to increase.
However, these applications of AI bring with them various challenges such as security and data privacy issues, bias, interpretability and explainability and ethical considerations. As AI becomes increasingly integrated into the healthcare industry, the significance and access granted to AI will play a pivotal role in achieving its complete potential and maximising benefits for the medical environment. For AI to function seamlessly, it relies on the continuous flow of comprehensive data and meaningful integration into clinical workflows. Further investment in the resources used to drive advancements in AI as well as transparency of the AI systems being integrated into the clinical environment is needed to help overcome the aforementioned challenges. This will not only foster trust between the public and AI systems but will also facilitate the accelerated development and utilisation of AI in various medical specialties.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-38/rc
Peer Review File: Available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-38/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-38/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shams M, Abdallah S, Alsadoun L, et al. Oncological Horizons: The Synergy of Medical and Surgical Innovations in Cancer Treatment. Cureus 2023;15:e49249. [Crossref] [PubMed]
- Ali O, Abdelbaki W, Shrestha A, et al. A systematic literature review of artificial intelligence in the healthcare sector: Benefits, challenges, methodologies, and functionalities. Journal of Innovation and Knowledge 2023;8:100333. [Crossref]
- Handley E. Intelligent triage: a vital step toward resolving the problem of capacity in NHS cancer services and cancer referrals [Internet]. Open Access Government. 2023 [cited 2024 Jan 17]. Available online: https://www.openaccessgovernment.org/intelligent-triage-capacity-nhs-cancer-services/155197/
- PinPoint Data Science [Internet]. [cited 2024 Jan 17]. FAQs. Available online: https://www.pinpointdatascience.com/faqs
- Gebrael G, Sahu KK, Chigarira B, et al. Enhancing Triage Efficiency and Accuracy in Emergency Rooms for Patients with Metastatic Prostate Cancer: A Retrospective Analysis of Artificial Intelligence-Assisted Triage Using ChatGPT 4.0. Cancers (Basel) 2023;15:3717. [Crossref] [PubMed]
- Sarbay İ, Berikol GB, Özturan İU. Performance of emergency triage prediction of an open access natural language processing based chatbot application (ChatGPT): A preliminary, scenario-based cross-sectional study. Turk J Emerg Med 2023;23:156-61. [Crossref] [PubMed]
- Xavier D, Miyawaki I, Campello Jorge CA, et al. Artificial intelligence for triaging of breast cancer screening mammograms and workload reduction: A meta-analysis of a deep learning software. J Med Screen 2023; Epub ahead of print. [Crossref] [PubMed]
- Alkatout I, Biebl M, Momenimovahed Z, et al. Has COVID-19 Affected Cancer Screening Programs? A Systematic Review. Front Oncol 2021;11:675038. [Crossref] [PubMed]
- Shur JD, Doran SJ, Kumar S, et al. Radiomics in Oncology: A Practical Guide. Radiographics 2021;41:1717-32. [Crossref] [PubMed]
- Lu H, Arshad M, Thornton A, et al. A mathematical-descriptor of tumor-mesoscopic-structure from computed-tomography images annotates prognostic- and molecular-phenotypes of epithelial ovarian cancer. Nat Commun 2019;10:764. [Crossref] [PubMed]
- Pei Q, Luo Y, Chen Y, et al. Artificial intelligence in clinical applications for lung cancer: diagnosis, treatment and prognosis. Clin Chem Lab Med 2022;60:1974-83. [Crossref] [PubMed]
- Chen BT, Chen Z, Ye N, et al. Differentiating Peripherally-Located Small Cell Lung Cancer From Non-small Cell Lung Cancer Using a CT Radiomic Approach. Front Oncol 2020;10:593. [Crossref] [PubMed]
- Lång K, Josefsson V, Larsson AM, et al. Artificial intelligence-supported screen reading versus standard double reading in the Mammography Screening with Artificial Intelligence trial (MASAI): a clinical safety analysis of a randomised, controlled, non-inferiority, single-blinded, screening accuracy study. Lancet Oncol 2023;24:936-44. [Crossref] [PubMed]
- Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci 2020;111:1452-60. [Crossref] [PubMed]
- Yeh MC, Wang YH, Yang HC, et al. Artificial Intelligence-Based Prediction of Lung Cancer Risk Using Nonimaging Electronic Medical Records: Deep Learning Approach. J Med Internet Res 2021;23:e26256. [Crossref] [PubMed]
- Liao W, Coupland CAC, Burchardt J, et al. Predicting the future risk of lung cancer: development, and internal and external validation of the CanPredict (lung) model in 19·67 million people and evaluation of model performance against seven other risk prediction models. Lancet Respir Med 2023;11:685-97. [Crossref] [PubMed]
- Callender T, Imrie F, Cebere B, et al. Assessing eligibility for lung cancer screening using parsimonious ensemble machine learning models: A development and validation study. PLoS Med 2023;20:e1004287. [Crossref] [PubMed]
- Howell D, Buttery R, Badrinath P, et al. Developing a risk prediction tool for lung cancer in Kent and Medway, England: cohort study using linked data. BJC Rep 2023;1:1-11. [Crossref]
- Ahmed KT, Sun J, Cheng S, et al. Multi-omics data integration by generative adversarial network. Bioinformatics 2021;38:179-86. [Crossref] [PubMed]
- Marakulina D, Vorontsov IE, Kulakovskiy IV, et al. EpiFactors 2022: expansion and enhancement of a curated database of human epigenetic factors and complexes. Nucleic Acids Res 2023;51:D564-70. [Crossref] [PubMed]
- Ahmed YW, Alemu BA, Bekele SA, et al. Epigenetic tumor heterogeneity in the era of single-cell profiling with nanopore sequencing. Clin Epigenetics 2022;14:107. [Crossref] [PubMed]
- Huang S, Yang J, Fong S, et al. Artificial intelligence in cancer diagnosis and prognosis: Opportunities and challenges. Cancer Lett 2020;471:61-71. [Crossref] [PubMed]
- Kim DW, Lee S, Kwon S, et al. Deep learning-based survival prediction of oral cancer patients. Sci Rep 2019;9:6994. [Crossref] [PubMed]
- Tong Z, Zhou Y, Wang J. Identifying potential drug targets in hepatocellular carcinoma based on network analysis and one-class support vector machine. Sci Rep 2019;9:10442. [Crossref] [PubMed]
- Ivanovic S, El-Kebir M. Modeling and predicting cancer clonal evolution with reinforcement learning. Genome Res 2023;33:1078-88. [Crossref] [PubMed]
- Jiang P, Sinha S, Aldape K, et al. Big data in basic and translational cancer research. Nat Rev Cancer 2022;22:625-39. [Crossref] [PubMed]
- Niida A, Mimori K, Shibata T, et al. Modeling colorectal cancer evolution. J Hum Genet 2021;66:869-78. [Crossref] [PubMed]
- Hickman SE, Payne NR, Black RT, et al. Mammography Breast Cancer Screening Triage Using Deep Learning: A UK Retrospective Study. Radiology 2023;309:e231173. [Crossref] [PubMed]
- Turabi KS, Deshmukh A, Paul S, et al. Drug repurposing-an emerging strategy in cancer therapeutics. Naunyn Schmiedebergs Arch Pharmacol 2022;395:1139-58. [Crossref] [PubMed]
- Cavasotto CN, Scardino V. Machine Learning Toxicity Prediction: Latest Advances by Toxicity End Point. ACS Omega 2022;7:47536-46. [Crossref] [PubMed]
- Lalmuanawma S, Hussain J, Chhakchhuak L. Applications of machine learning and artificial intelligence for Covid-19 (SARS-CoV-2) pandemic: A review. Chaos Solitons Fractals 2020;139:110059. [Crossref] [PubMed]
- Celebi R, Bear Don't Walk O 4th, Movva R, et al. In-silico Prediction of Synergistic Anti-Cancer Drug Combinations Using Multi-omics Data. Sci Rep 2019;9:8949. [Crossref] [PubMed]
- Yang H, Sun L, Li W, et al. In Silico Prediction of Chemical Toxicity for Drug Design Using Machine Learning Methods and Structural Alerts. Front Chem 2018;6:30. Erratum in: Front Chem 2018;6:129. [Crossref] [PubMed]
- Monzel AS, Hemmer K, Kaoma T, et al. Machine learning-assisted neurotoxicity prediction in human midbrain organoids. Parkinsonism Relat Disord 2020;75:105-9. [Crossref] [PubMed]
- Kavalci E, Hartshorn A. Improving clinical trial design using interpretable machine learning based prediction of early trial termination. Sci Rep 2023;13:121. [Crossref] [PubMed]
- Huang C, Clayton EA, Matyunina LV, et al. Machine learning predicts individual cancer patient responses to therapeutic drugs with high accuracy. Sci Rep 2018;8:16444. [Crossref] [PubMed]
- Chow R, Midroni J, Kaur J, et al. Use of artificial intelligence for cancer clinical trial enrollment: a systematic review and meta-analysis. J Natl Cancer Inst 2023;115:365-74. [Crossref] [PubMed]
- Dara S, Dhamercherla S, Jadav SS, et al. Machine Learning in Drug Discovery: A Review. Artif Intell Rev 2022;55:1947-99. [Crossref] [PubMed]
- Wang Y, Feng W. Cancer-related psychosocial challenges. Gen Psychiatr 2022;35:e100871. [Crossref] [PubMed]
- Pan LC, Wu XR, Lu Y, et al. Artificial intelligence empowered digital health technologies in cancer survivorship care: A scoping review. Asia Pac J Oncol Nurs 2022;9:100127. [Crossref] [PubMed]
- Bibault JE, Chaix B, Nectoux P, et al. Healthcare ex Machina: Are conversational agents ready for prime time in oncology? Clin Transl Radiat Oncol 2019;16:55-9. [Crossref] [PubMed]
- Tzelves L, Manolitsis I, Varkarakis I, et al. Artificial intelligence supporting cancer patients across Europe-The ASCAPE project. PLoS One 2022;17:e0265127. [Crossref] [PubMed]
- Shreve JT, Khanani SA, Haddad TC. Artificial Intelligence in Oncology: Current Capabilities, Future Opportunities, and Ethical Considerations. Am Soc Clin Oncol Educ Book 2022;42:1-10. [Crossref] [PubMed]
- Hassija V, Chamola V, Mahapatra A, et al. Interpreting Black-Box Models: A Review on Explainable Artificial Intelligence. Cognitive Computation 2024;16:45-74. [Crossref]
- Nakayama LF, Mitchell WG, Ribeiro LZ, et al. Fairness and generalisability in deep learning of retinopathy of prematurity screening algorithms: a literature review. BMJ Open Ophthalmol 2023;8:e001216. [Crossref] [PubMed]
- Pagano TP, Loureiro RB, Lisboa FVN, et al. Bias and Unfairness in Machine Learning Models: A Systematic Review on Datasets, Tools, Fairness Metrics, and Identification and Mitigation Methods. Big Data Cogn Comput 2023;7:15. [Crossref]
Cite this article as: Al Hajji Y, Al Hajji FZ, Lee LYW. Navigating the horizon of opportunity: a comprehensive review of artificial intelligence applications in cancer care—insights from the 2024 landscape, a narrative review. J Med Artif Intell 2024;7:38.


