
Machine learning approaches for DAS28 score prediction after rituximab treatment in rheumatoid arthritis patients
Highlight box
Key findings
• This study presents an approach for developing machine learning (ML) models aimed at predicting treatment response to rituximab in rheumatoid arthritis patients by analyzing their clinical profiles, including demographic data, clinical features, laboratory characteristics, and FCGR3A genotyping. This approach aids in predicting the disease activity and treatment response after rituximab initiation that could be useful in guiding clinical decisions, enhancing healthcare accuracy and efficiency.
What is known and what is new?
• Despite the effectiveness of biological disease-modifying antirheumatic drugs in the treatment of rheumatoid arthritis, 30–40% of patients do not achieve the treatment goal and only 50% achieve a sustained response. Many studies have examined the impact of demographic and clinical factors commonly assumed in routine clinical practice, such as gender, age, disease duration, disease activity, and comorbidities on patients’ responses to biological disease-modifying antirheumatic treatment. Consequently, there is a growing interest in using ML methods to evaluate the response to biological disease-modifying antirheumatic treatment.
• To the authors’ knowledge, there is a research gap in predicting disease activity using Disease Activity Score in 28 Joints scores at 6 months and 1 year after rituximab treatment in rheumatoid arthritis patients, particularly by utilizing easily assessable clinical variables commonly used in routine practice, along with FCGR3A genotyping.
What is the implication, and what should change now?
• ML methods for predicting treatment response to rituximab should be investigated with larger population groups.
Introduction
Background
Rheumatoid arthritis is characterized by inflammation in the synovial tissue, and if not properly treated, it can cause significant damage to joints, potentially leading to severe disability.
The management of rheumatoid arthritis requires careful consideration of treatment options due to the significant variation in disease progression and patient variability (1). The goal of treatment is to reduce and manage disease activity in order to prevent progressive joint damage and loss of physical function. Decisions about treatment are based on assessing disease activity regularly and consistently evaluating the patient’s clinical condition (2).
The treat-to-target strategy, recommended in the European Alliance of Associations for Rheumatology (EULAR) guideline, aims for at least 50% improvement in disease activity within the first 3 months. It also targets achieving the main treatment target, which is remission in early and low disease activity in long-standing disease within approximately 6 months (3). Biologic disease-modifying anti-rheumatic drugs (bDMARDs) are often considered a promising treatment for patients whose disease remains active despite previous treatment with conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) (4). Despite the effectiveness of bDMARDS in the treatment of rheumatoid arthritis, 30–40% of patients do not achieve the treatment goal and only 50% achieve a sustained response (5,6). Many studies have examined the impact of demographic and clinical factors, such as gender, age, disease duration, disease activity, and comorbidities on patients’ responses to bDMARD treatment (7-10). Using patient characteristics to accurately predict disease activity after treatment initiation may help clinicians in optimizing treatment management, preventing serious side effects, and reducing unnecessary healthcare costs.
Rituximab is a biologic therapy approved for the management of severe active rheumatoid arthritis in adults unresponsive to csDMARDs. Studies have suggested that various patient characteristics are associated with the response to rituximab, including clinical factors such as baseline Disease Activity Score in 28 Joints (DAS28), disease duration, steroid use, biochemical markers like rheumatoid factor (RF), anti-cyclic citrullinated peptide (anti-CCP) positivity, and immunoglobulin levels (11,12). Our previous study, conducted with a smaller sample size, showed that persistently active rheumatoid arthritis disease and DAS28 using erythrocyte sedimentation rate (DAS28-ESR), with higher scores at baseline and 3 months after treatment onset are associated with lower rituximab response chances at 6 months (13). Additionally, several studies showed that polymorphisms in the gene encoding the Fc receptor 3A (FCGR3A), which impact its affinity for the Fc region, have been linked to the effectiveness of rituximab (14-18).
Rationale and knowledge gap
As medical data continues to grow, new artificial intelligence (AI) tools are becoming available for diagnosing, predicting outcomes, or suggesting treatments in clinical practice. In rheumatic and musculoskeletal diseases, machine learning (ML) is the primary focus of AI applications (19). Consequently, there is a growing interest in using ML to evaluate therapeutic responses with bDMARDs, as evidenced by recent studies (20-25). To our knowledge, there is a gap in research regarding the prediction of disease activity using DAS28 scores at 6 months and 1 year after rituximab treatment in patients with rheumatoid arthritis.
Objective
The goal of this study was to utilize ML models to predict DAS28 scores 6 months and 1 year following rituximab treatment, based on patient clinical characteristics. We identified the most relevant baseline clinical features and their effects on rituximab treatment outcomes. This approach aids in predicting the disease activity and treatment response after rituximab initiation, enhancing healthcare accuracy and efficiency. We present this article in accordance with the TRIPOD reporting checklist (available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-288/rc).
Methods
Study design
This is a retrospective cohort study aimed to develop ML prediction models that estimate disease activity, defined by the DAS28 score 6 months, and 1 year after rituximab treatment initiation in patients with rheumatoid arthritis by analyzing their clinical, biochemical, and genetic data. The study population comprised of 100 rheumatoid arthritis patients who received rituximab treatment at the University Clinic of Rheumatology in North Macedonia between 2018 and 2023. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval was granted by the local Ethics Committee of Faculty of Pharmacy, Ss. Cyril and Methodius University, Skopje, R.N. Macedonia, who agreed that the findings in this report were based on normal clinical practice and therefore were suitable for dissemination (No. 02-284/4), and individual consent for this retrospective analysis was waived.
Population
Individuals aged 18 years and above, who were admitted to the University Clinic of Rheumatology with a diagnosis of active rheumatic disease and treated with rituximab, were eligible for inclusion in the study. The patients were diagnosed by rheumatologists at the University Rheumatology Clinic in Skopje based on the American College of Rheumatology (ACR)/EULAR 2010 criteria (26). All patients had active disease at the treatment onset, defined by the disease activity score in DAS28-ESR, and had previously undergone unsuccessful therapy with at least one DMARD (conventional synthetic or biologic). The rituximab doses administered were 2×500 and 2×1,000 mg (depending on the physician decision) via intravenous infusion on days 1 and 15, with repeated courses of therapy at least 6 months afterwards, depending on the clinical response. Informed consent was obtained from all individual participants included in the study.
Data sources and collection
Patient demographics, clinical characteristics, and treatment specifics were obtained from the national database “MojTermin”. At the start of the rituximab treatment, baseline data included age, gender, disease duration, history of past and current anti-rheumatic therapies, and assessments of disease activity such as DAS28 score, Health Assessment Questionnaire (HAQ), ESR, and C-reactive protein (CRP) levels. Moreover, baseline serological data on RF and anti-CCP antibodies were recorded. Follow-up assessments of patients after the initial rituximab infusion were conducted over a period ranging from 3 to 6 months. For molecular analyses DNA was extracted from peripheral blood samples using a MagCore Genomic DNA Whole Blood Kit on the automated MagCore Nucleic Acid extractor and its concentration was determined by assessing ultraviolet (UV) absorption at 260 nm using the Nanodrop 2000 spectrophotometer. Genotyping of the single nucleotide polymorphism (rs396991) was done using specific TaqMan assays (Thermo Fisher Scientific, Waltham, MA, USA). Real-time polymerase chain reaction (PCR) was performed with allele discrimination method on Stratagene Mx3005P instrument (Agilent Technologies, Santa Clara, CA, USA). The PCR was carried out using 50 ng of genomic DNA in a total volume of 11.5 µL in accordance with the manufacturer protocol instructions. Thermal cycling conditions were as follows: initial denaturation at 95 ℃ for 10 min, 45 cycles consisted of denaturation at 95 ℃ for 10 s, and annealing/extension at 60 ℃ for 1 min 30 s. The obtained results were analyzed with MxPro Software.
Statistical analysis
Patient data was statistically processed with the International Business Machines Corporation Statistical Package for the Social Sciences (IBM SPSS) version 26. Correlation tests were used to examine the associations between variables and DAS28 score at 6 months. For continuous variables, Pearson correlation and paired sample t-tests were applied, while Kendall’s tau-b (τb) was used for correlations involving ordinal variables and combinations of ordinal and continuous variables. All tests were performed with a confidence level of 95%.
ML methodology
The dataset contains multiple measurements of DAS28 throughout the treatment period, i.e., values are measured at patient admission before treatment is applied, then values are measured after treatment rounds at 3 and 6 months, and between the first and second year after treatment. The DAS28 measured before treatment is used as a reference point for understanding patients’ response to treatment and as such was considered as input information for the model to learn from. On the other hand, the remaining measurements are the patient’s actual response to treatment and are therefore subject to prediction. Namely, our approach analyses whether ML algorithms can estimate the DAS28 values measured at 6 months, and after 1 year based on patient clinical information. Missing data for characteristics are replaced with −1 to signify the absence of information, we treat this as informative, while opting to drop data for DAS28 scores.
The following regression algorithms were trained to predict DAS28 values at 6 months, and after 1 year respectively, i.e., Linear Regression, Support Vector Regression (SVR), Cat Boost Regressor, Elastic Net, and Extreme Gradient Boosting (XGB) Regressor. The performance of the models was evaluated using different input variable combinations in a 5-fold cross-validation setting, with training and testing data being divided into 20–80% ratio per cross-validation. Cross-validation allowed the models to be trained and tested five times. This was accomplished by dividing the data into 5 folds. In each of the five iterations 4 folds were used to train the models and 1-fold was used to test the model. As each new iteration takes a different fold for testing the model, subsequently the training data used were the remaining 4 folds. The results were averaged across all five tests. With this we ensured that each individual patient was considered as a test subject exactly once throughout the process. It is standard that ML approaches use the holdout technique, where data is divided into train data for model training and test data for model evaluation. However, the holdout technique has one significant drawback, where the result is dependent on the test data and its distribution. This is not an issue with large datasets. However, in cases of small datasets (as here) the test data selection can make or break the model, i.e., the same model can perform extremely well for one test data and extremely badly for another. As k-fold cross-validation addresses this issue by creating an average across k-fold, it provides better indication of how well the models will perform on unseen data and thus became our chosen approach. All ML models were generated and evaluated using Python code (Python version 3.8.8). Scikit-learn library was used for Linear Regression, SVR, Elastic Net, while additionally the XGBoost library was used for the XGB Regressor models, and the CatBoost library was used for the Cat Boost Regressor model. The prediction models were evaluated with custom metrics adjusted to fit the purpose of our research, i.e., we observe the average difference between actual and predicted DAS28 values, and the number of patients where the precited DAS28 value group deviates from the actual DAS28 value group. The considered DAS28 score value groups are as follows: remission (<2.6), low activity (score ≥2.6 and <3.1), moderate activity (score ≥3.1 and <5.1), and high activity (score ≥5.1).
Results
Overview of patient information
An overview of the patient clinical variables from the data is given in Table 1, with input variables organized in four different categories (demographic information, clinical parameters, laboratory measurements, and FCGR3A genotypes), and the two individual output variables. Furthermore, the table details information on the variable values and their distribution, with categorical values represented in percentages and continuous values accompanied with an averaged representation. Table 2 presents the association between patient characteristics and DAS28 scores 6 months after rituximab treatment.
Table 1
| Variable name | Data (n=100) |
|---|---|
| Model input variables | |
| Demographics | |
| Gender, female | 86 (86.0) |
| Age (years) | 56±8 |
| Clinical parameters | |
| Disease time (years) | 11±7 |
| Disease activity, moderately active | 48 (48.0) |
| Treatment dose with rituximab, 2×500 mg | 26 (26.0) |
| HAQ score | 1.8±0.6 |
| Laboratory measurements | |
| CRP (mg/L) | 20±32 |
| Anti-CCP (EU/mL) | 134±222 |
| RF (IU/mL) | 88±132 |
| ESR (mm/h) | 40±27 |
| CRP at 6 months (mg/L) | 9±20 |
| Anti-CCP at 6 months (EU/mL) | – |
| RF at 6 months (IU/mL) | 26±61 |
| ESR at 6 months (mm/h) | 25±24 |
| DAS28-ESR before treatment initiation | 5.4±0.9 |
| FCGR3A genotypes† | |
| VV | 21 (21.4) |
| VF | 51 (52.0) |
| FF | 26 (26.5) |
| Model output variables | |
| DAS28 at 6 months | 3.9±1.0 |
| DAS28 after 1 year | 3.3±2.4 |
Data are presented as n (%) or mean ± standard deviation. †, valid data was available for 98 patients. Moderate activity: DAS28 score ≥3.1 and <5.1. HAQ, Health Assessment Questionnaire; CRP, C-reactive protein; anti-CCP, anti-cyclic citrullinated peptide; RF, rheumatoid factor; ESR, erythrocyte sedimentation rate; DAS28, Disease Activity Score in 28 Joints.
Table 2
| Variables | Correlation coefficient | P value |
|---|---|---|
| Patient characteristics† | ||
| Gender | 0.332** | 0.001 |
| Persistence of disease activity | −0.202* | 0.04 |
| Rituximab dosage | −0.006 | 0.95 |
| Age | 0.045 | 0.58 |
| Disease duration | 0.114 | 0.17 |
| DAS28 at the baseline | −0.094 | 0.25 |
| DAS28 after 3 months | −0.369** | <0.001 |
| CRP | −0.034 | 0.68 |
| ESR | −0.099 | 0.22 |
| HAQ | 0.105 | 0.21 |
| Additional therapy‡ | ||
| Sulfasalazine | 0.184 | 0.06 |
| Prednisolone | 0.133 | 0.18 |
| Leflunomide | 0.000 | >0.99 |
| Chloroquine | −0.065 | 0.52 |
| Methotrexate | −0.137 | 0.17 |
| Hydroxychloroquine | −0.035 | 0.73 |
| Azathioprine | −0.158 | 0.11 |
| Status of RF and anti-CCP§ | ||
| RF | 0.844 | 0.40 |
| Anti-CCP | 3.257 | 0.002 |
| RF/anti-CCP | 4.552 | <0.001 |
†, Kendall’s tau-b; ‡, Pearson correlation; §, paired samples t-test. *, correlation is significant at the 0.05 level (two-tailed); **, correlation is significant at the 0.01 level (two-tailed). DAS28, Disease Activity Score in 28 Joints; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptide.
As Table 1 shows, there exists an overall improvement in DAS28 for the entire patient cohort with each treatment passed, i.e., the overall DAS28 before treatment averages 5.4, whereas DAS28 after treatment is 4.1, 3.9, and 3.3 at 3 months, 6 months, and after 1 year, respectively. The data is predominantly collected from patients with moderate to high activity of the disease, i.e., with DAS28 values before treatment ≥3.1.
Table 1 further illustrates the specifics of the cohort. The cohort consists of predominantly female patients (86%), with an average age of 56 years and a standard deviation of 8 years. Most patients have had the condition for many years, shown by the average duration of 11 years with a standard deviation of 7 years. Moreover, in 48% of patients the disease is moderately active, whilst in the remaining 52% the disease is highly active; 26% of patients were treated with 2×500 mg dose, and the remaining 74% were treated with 2×1,000 mg dose of rituximab. CRP, RF, and ESR measurements are taken both before treatment initiation and at 6 months. However, anti-CCP measurements are not routinely performed at 6 months, so valid anti-CCP data is only available prior to the start of treatment. The distribution of patients with RF and anti-CCP positivity according to the DAS-based EULAR response criteria (27) (good or moderate) at 6 months was: response in 37/51 (72.5%) anti-CCP positive patients, 52/67 (77.6%) RF positive patients, and 32/43 (74.4%) RF-positive/anti-CCP positive patients. The distribution of FCGR3A genotypes was VF (52%), VV (21.4%) and FF (26.5%). The distribution of FCGR3A genotypes according to the DAS-based EULAR response criteria (good or moderate) at 6 months was as follows [response in 41/51 (80.4%) VF, 16/21 (76.2%) VV, and 16/26 (61.5%) FF patients].
ML results
Four different variable sets were used for training the models in predicting DAS28 measurements. The first and second set of variables focus on the data obtained from patients before re-treatment and re-testing. Both sets consider the DAS28 value before treatment, the HAQ score, laboratory measurements taken at the beginning of the treatment process, disease duration and activity and the rituximab dose given to patients. The difference between the first and second set of variables is that in the first set in addition to the above-listed variables demographic information, age, and gender, were also considered. The third and fourth set of variables expand on the first and second set with the laboratory measurements obtained at 6 months. Similarly, to the first and second set, the difference between the third and fourth set is that the third set includes demographic information, whilst the fourth set does not. The first and second set of variables were used for predicting DAS28 at 6 months, and a year after treatment, whilst the third and fourth set of variables were used for predicting DAS28 a year after treatment since they include variables measured at 6 months. The obtained results are presented in Table 3.
Table 3
| Variables set | Linear Regression | SVR | Cat Boost Regressor | Elastic Net | XGB Regressor | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVG | CNT | R2 score | AVG | CNT | R2 score | AVG | CNT | R2 score | AVG | CNT | R2 score | AVG | CNT | R2 score | |||||
| Patient characteristic variables set† | |||||||||||||||||||
| Variable set 1 | 0.7±0.3 | 6.8±2.7 | 0.25 | 0.8±0.1 | 7.2±2.2 | 0.22 | 0.7±0.1 | 7.8±3.2 | 0.48 | 0.9±0.2 | 8±2 | 0.18 | 0.7±0.1 | 9±4 | 0.35 | ||||
| Variable set 2 | 0.8±0.3 | 6.4±2.7 | 0.20 | 0.8±0.1 | 7.2±2.2 | 0.25 | 0.7±0.1 | 7.8±2.7 | 0.46 | 0.9±0.2 | 8±2.5 | 0.18 | 0.7±0.1 | 8.4±3.9 | 0.38 | ||||
| Patient characteristic variables set‡ | |||||||||||||||||||
| Variable set 1 | 0.9±0.1 | 7.2±1.7 | 0.14 | 0.8±0.1 | 4.6±1.1 | 0.20 | 0.7±0.2 | 4.6±1.6 | 0.26 | 0.8±0.1 | 4.4±1.1 | 0.25 | 0.8±0.2 | 5±1.4 | 0.16 | ||||
| Variable set 2 | 0.9±0.2 | 7.8±1.4 | 0.12 | 0.8±0.1 | 4.6±1.1 | 0.22 | 0.7±0.2 | 4.8±1.9 | 0.29 | 0.8±0.1 | 4.4±1.1 | 0.26 | 0.8±0.2 | 5.4±2.7 | 0.12 | ||||
| Variable set 3 | 0.9±0.2 | 7.8±1.7 | 0.25 | 0.8±0.1 | 4.6±1.1 | 0.34 | 0.6±0.2 | 4.8±1.7 | 0.61 | 0.8±0.1 | 4.4±1.1 | 0.30 | 0.6±0.2 | 5±2.8 | 0.44 | ||||
| Variable set 4 | 0.9±0.2 | 7.4±1.9 | 0.28 | 0.8±0.1 | 4.6±1.1 | 0.34 | 0.67±0.2 | 4.4±2.3 | 0.58 | 0.8±0.1 | 4.4±1.1 | 0.29 | 0.6±0.2 | 4.6±2.4 | 0.47 | ||||
Data are presented as mean ± standard deviation. †, used to predict DAS28 6 months after rituximab treatment; ‡, used to predict DAS28 1 year after rituximab treatment. SVR, Support Vector Regression; XGB, Extreme Gradient Boosting; AVG, average difference between actual and predicted DAS28 values; CNT, the number of patients where the predicted DAS28 value group deviates from the actual DAS28 value group; DAS28, Disease Activity Score in 28 Joints.
Discussion
Key findings
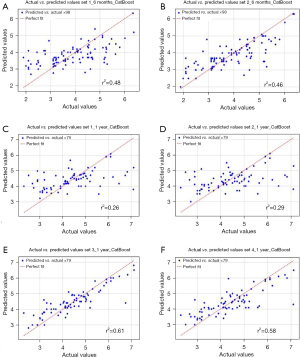
In the current EULAR rheumatoid arthritis management recommendations, clinical remission or at least low disease activity is the ideal target with adjustment of therapeutic strategies if there is no improvement at 3 months or if the treatment target is not achieved at 6 months (3). The DAS28-ESR value is a key component in defining disease activity as a target for achieving treatment goals and is the most suitable tool for assessing disease activity in the Macedonian clinical setting. Therefore, we believe that predicting DAS28 scores will enhance clinical decision for treatment management. By developing ML models to predict DAS28 values, we identified key clinical features linked to the response to rituximab treatment. Among these models, the Cat Boost Regressor model achieved the best overall performance across all ML experiments, while the Linear Regression model exhibited the worst performance, as demonstrated by the average error between predicted and actual DAS28 values. Moreover, the best performance from the Cat Boost Regressor can be observed for variable sets 3 and 4 when predicting DAS28 after 1 year, with an average error between predicted and actual values reduced to 0.6±0.2, and with roughly 4 to 7 patients per fold being predicted outside of their corresponding disease severity ranges, which is indicative of the benefit of providing the model with additional laboratory analysis at the 6th-month interval. Figure 1 presents the scatter plots, demonstrating that the Cat Boost Regressor model achieved its highest coefficient of determination (r2) when predicting the 1-year response using variables measured at 6 months. However, in the case of predicting DAS28 at year 1, when observing the count of patients in the corresponding DAS28 severity group the performance of SVR and Elastic Net was comparable—and, in cases, better—than that of the Cat Boost Regressor. Since precedence is given to the regressive power of the model, we observe the averaged error and find Cat Boost Regressor as the best performing model overall. Future work could include an ensemble model which utilizes the best performance aspects of each individual model.
Strengths and limitations
In our study, the most significant predictors of response to rituximab therapy were gender, DAS28 value at 3 months, and seropositivity for anti-CCP and RF. Additionally, we included FCGR3A genotyping, which is associated with variations in rituximab treatment outcomes. By incorporating this genotyping, we aim to enhance the predictive power, providing clinicians with a more comprehensive tool for decision-making and ensuring more personalized treatment for each patient. Additionally, our results showed that incorporating laboratory analyses obtained at month 6 enhances the models’ performance in predicting DAS28 after 1 year of treatment and improves precision. The prediction models were evaluated with custom metrics. Our custom metrics were designed to be more accessible to medical personnel who may not be familiar with the statistical aspects of machine learning models. Therefore, even though we only perform regression analysis, we give an average difference between the actual and predicted values for patients along with the number of patients which were predicted outside their severity ranges. This study has several limitations. The main limitation is the sample size which is rather small compared with data amount usually used in ML. The number of patients and the complexity of the disease, along with the exclusion of factors such as environment, lifestyle, and non-pharmacological treatments from the models, limit the accuracy of the algorithms and make it difficult to draw strong conclusions. Additionally, the single-center design of the study may reduce the generalizability of the findings, and FCGR3A genotyping datasets are not available for every patient and are expensive for healthcare systems.
Comparison with similar researches
To develop these models, we utilized patient’s characteristics commonly assumed in routine clinical practice, except for FCGR3A genotyping. This approach aligns with findings from other studies where ML models such as least absolute shrinkage and selection operator (LASSO), Ridge, Support Vector Machine, Random Forest, and XGBoost were developed based on easily assessable clinical variables in routine clinical practice and showed predictive power for treatment response with bDMARDs. The most significant characteristics used to develop these models that were associated with predicting response to therapy were RF, ESR, initial DAS28-ESR value, disease duration, and CRP (28-31).
Explanations of findings
Timely and effective treatment with rituximab for rheumatoid arthritis, is essential to avoid disease progression. We have developed ML models capable of predicting changes in DAS28 score at 6 months, and 1 year after rituximab treatment for patients with rheumatoid arthritis, utilizing their clinical profiles laboratory data and FCGR3A genotype. By development of ML models to predict DAS28 scores at various intervals post-rituximab treatment, our goal is to offer clinicians a complete tool for making decisions, ensuring that each patient receives the most appropriate and effective treatment possible. These models enable personalized therapy, optimizing rituximab treatment schedules to improve patient outcomes while minimizing costs and potential side effects.
Implications and actions needed
We intend to estimate the performance of classic ML algorithms and their performance on small patient populations with the hopes of extending the dataset in the future and reapplying our approach to more diverse data. Our idea was to analyze the most significant predictors but to also develop a pipeline which will assist medical personnel in future therapy predictions and make the treatment readily available when necessary. Subsequent studies should validate these predictive models across different settings and with larger patient groups to confirm their applicability and improve their predictive power.
Conclusions
We successfully developed ML models to predict treatment response to rituximab in RA patients by analyzing their clinical profiles. Our findings indicate that advanced ML techniques could be valuable in guiding clinical decisions, potentially improving treatment outcomes for RA patients using rituximab.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-288/rc
Data Sharing Statement: Available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-288/dss
Peer Review File: Available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-288/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jmai.amegroups.com/article/view/10.21037/jmai-24-288/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by local Ethics Committee of Faculty of Pharmacy, Ss. Cyril and Methodius University, Skopje, R.N. Macedonia (No. 02-284/4) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Radu AF, Bungau SG. Management of Rheumatoid Arthritis: An Overview. Cells 2021;10:2857. [Crossref] [PubMed]
- Guo Q, Wang Y, Xu D, et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 2018;6:15. [Crossref] [PubMed]
- Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 2023;82:3-18. [Crossref] [PubMed]
- Mysler E, Caubet M, Lizarraga A. Current and Emerging DMARDs for the Treatment of Rheumatoid Arthritis. Open Access Rheumatol 2021;13:139-52. [Crossref] [PubMed]
- Pierreisnard A, Issa N, Barnetche T, et al. Meta-analysis of clinical and radiological efficacy of biologics in rheumatoid arthritis patients naive or inadequately responsive to methotrexate. Joint Bone Spine 2013;80:386-92. [Crossref] [PubMed]
- Combe B, Rincheval N, Benessiano J, et al. Five-year favorable outcome of patients with early rheumatoid arthritis in the 2000s: data from the ESPOIR cohort. J Rheumatol 2013;40:1650-7. [Crossref] [PubMed]
- Novella-Navarro M, Plasencia C, Tornero C, et al. Clinical predictors of multiple failure to biological therapy in patients with rheumatoid arthritis. Arthritis Res Ther 2020;22:284. [Crossref] [PubMed]
- Khader Y, Beran A, Ghazaleh S, et al. Predictors of remission in rheumatoid arthritis patients treated with biologics: a systematic review and meta-analysis. Clin Rheumatol 2022;41:3615-27. [Crossref] [PubMed]
- Wijbrandts CA, Tak PP. Prediction of Response to Targeted Treatment in Rheumatoid Arthritis. Mayo Clin Proc 2017;92:1129-43. [Crossref] [PubMed]
- Cordisco AJ, Olave M, George MD, et al. Identifying Factors Associated With Treatment Response in Rheumatoid Arthritis Clinical Trials. ACR Open Rheumatol 2022;4:811-8. [Crossref] [PubMed]
- Couderc M, Mathieu S, Pereira B, et al. Predictive factors of rituximab response in rheumatoid arthritis: results from a French university hospital. Arthritis Care Res (Hoboken) 2013;65:648-52. [Crossref] [PubMed]
- Łosińska K, Wilk M, Pripp AH, et al. Long-term drug effectiveness and survival for reference rituximab in rheumatoid arthritis patients in an ordinary outpatient clinic. Sci Rep 2022;12:8283. [Crossref] [PubMed]
- Mihajloska E, Dimkovski A, Grozdanova A, et al. Early predictive factors in routine clinical practice for rituximab therapy response in patients with rheumatoid arthritis. Reumatologia 2024;62:150-6. [Crossref] [PubMed]
- Jiménez Morales A, Maldonado-Montoro M, Martínez de la Plata JE, et al. FCGR2A/FCGR3A Gene Polymorphisms and Clinical Variables as Predictors of Response to Tocilizumab and Rituximab in Patients With Rheumatoid Arthritis. J Clin Pharmacol 2019;59:517-31. [Crossref] [PubMed]
- Pál I, Szamosi S, Hodosi K, et al. Effect of Fcγ-receptor 3a (FCGR3A) gene polymorphisms on rituximab therapy in Hungarian patients with rheumatoid arthritis. RMD Open 2017;3:e000485. [Crossref] [PubMed]
- Ruyssen-Witrand A, Rouanet S, Combe B, et al. Fcγ receptor type IIIA polymorphism influences treatment outcomes in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis 2012;71:875-7. [Crossref] [PubMed]
- Quartuccio L, Fabris M, Pontarini E, et al. The 158VV Fcgamma receptor 3A genotype is associated with response to rituximab in rheumatoid arthritis: results of an Italian multicentre study. Ann Rheum Dis 2014;73:716-21. [Crossref] [PubMed]
- Robinson JI, Md Yusof MY, Davies V, et al. Comprehensive genetic and functional analyses of Fc gamma receptors influence on response to rituximab therapy for autoimmunity. EBioMedicine 2022;86:104343. [Crossref] [PubMed]
- Kedra J, Radstake T, Pandit A, et al. Current status of use of big data and artificial intelligence in RMDs: a systematic literature review informing EULAR recommendations. RMD Open 2019;5:e001004. [Crossref] [PubMed]
- Lee S, Kang S, Eun Y, et al. Machine learning-based prediction model for responses of bDMARDs in patients with rheumatoid arthritis and ankylosing spondylitis. Arthritis Res Ther 2021;23:254. [Crossref] [PubMed]
- Sidey-Gibbons JAM, Sidey-Gibbons CJ. Machine learning in medicine: a practical introduction. BMC Med Res Methodol 2019;19:64. [Crossref] [PubMed]
- Turki T, Wang JTL. Clinical intelligence: New machine learning techniques for predicting clinical drug response. Comput Biol Med 2019;107:302-22. [Crossref] [PubMed]
- Guan Y, Zhang H, Quang D, et al. Machine Learning to Predict Anti-Tumor Necrosis Factor Drug Responses of Rheumatoid Arthritis Patients by Integrating Clinical and Genetic Markers. Arthritis Rheumatol 2019;71:1987-96. [Crossref] [PubMed]
- Sutcliffe M, Radley G, Barton A. Personalized medicine in rheumatic diseases: how close are we to being able to use genetic biomarkers to predict response to TNF inhibitors? Expert Rev Clin Immunol 2020;16:389-96. [Crossref] [PubMed]
- Bouget V, Duquesne J, Hassler S, et al. Machine learning predicts response to TNF inhibitors in rheumatoid arthritis: results on the ESPOIR and ABIRISK cohorts. RMD Open 2022;8:e002442. [Crossref] [PubMed]
- Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford) 2012;51:vi5-9. [Crossref] [PubMed]
- Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 2005;23:S93-9. [PubMed]
- Koo BS, Eun S, Shin K, et al. Machine learning model for identifying important clinical features for predicting remission in patients with rheumatoid arthritis treated with biologics. Arthritis Res Ther 2021;23:178. [Crossref] [PubMed]
- Ukalovic D, Leeb BF, Rintelen B, et al. Prediction of ineffectiveness of biological drugs using machine learning and explainable AI methods: data from the Austrian Biological Registry BioReg. Arthritis Res Ther 2024;26:44. [Crossref] [PubMed]
- Duong SQ, Crowson CS, Athreya A, et al. Clinical predictors of response to methotrexate in patients with rheumatoid arthritis: a machine learning approach using clinical trial data. Arthritis Res Ther 2022;24:162. [Crossref] [PubMed]
- Salehi F, Lopera Gonzalez LI, Bayat S, et al. Machine Learning Prediction of Treatment Response to Biological Disease-Modifying Antirheumatic Drugs in Rheumatoid Arthritis. J Clin Med 2024;13:3890. [Crossref] [PubMed]
Cite this article as: Mihajloska E, Velichkovska B, Dimkovski A, Staninova Stojovska M, Vasilevska A, Antova D, Shuminoski T, Grozdanova A, Suturkova L. Machine learning approaches for DAS28 score prediction after rituximab treatment in rheumatoid arthritis patients. J Med Artif Intell 2025;8:46.


